Bio-Functionalized Chitosan for Bone Tissue Engineering
- PMID: 34072888
- PMCID: PMC8198664
- DOI: 10.3390/ijms22115916
Bio-Functionalized Chitosan for Bone Tissue Engineering
Abstract
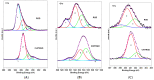
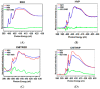
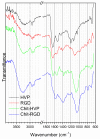
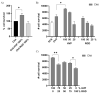
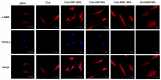
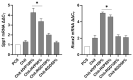
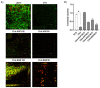
Hybrid biomaterials allow for the improvement of the biological properties of materials and have been successfully used for implantology in medical applications. The covalent and selective functionalization of materials with bioactive peptides provides favorable results in tissue engineering by supporting cell attachment to the biomaterial through biochemical cues and interaction with membrane receptors. Since the functionalization with bioactive peptides may alter the chemical and physical properties of the biomaterials, in this study we characterized the biological responses of differently functionalized chitosan analogs. Chitosan analogs were produced through the reaction of GRGDSPK (RGD) or FRHRNRKGY (HVP) sequences, both carrying an aldehyde-terminal group, to chitosan. The bio-functionalized polysaccharides, pure or "diluted" with chitosan, were chemically characterized in depth and evaluated for their antimicrobial activities and biocompatibility toward human primary osteoblast cells. The results obtained indicate that the bio-functionalization of chitosan increases human-osteoblast adhesion (p < 0.005) and proliferation (p < 0.005) as compared with chitosan. Overall, the 1:1 mixture of HVP functionalized-chitosan:chitosan is the best compromise between preserving the antibacterial properties of the material and supporting osteoblast differentiation and calcium deposition (p < 0.005 vs. RGD). In conclusion, our results reported that a selected concentration of HVP supported the biomimetic potential of functionalized chitosan better than RGD and preserved the antibacterial properties of chitosan.
Keywords: Chit-HVP; Chit-RGD; NEXAFS; XPS; chitosan; functionalization; h-osteoblasts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Bagheri-Khoulenjani S., Taghizadeh S., Mirzadeh H. An Investigation on the Short-Term Biodegradability of Chitosan with Various Molecular Weights and Degrees of Deacetylation. Carbohydr. Polym. 2009;78:773–778. doi: 10.1016/j.carbpol.2009.06.020. - DOI
-
- Palma P.J., Ramos J., Martins J.B., Diogenes A., Figueiredo M.H., Ferreira P., Viegas C., Santos J.M. Histologic Evaluation of Regenerative Endodontic Procedures with the Use of Chitosan Scaffolds in Immature Dog Teeth with Apical Periodontitis. J. Endod. 2017;43:1279–1287. doi: 10.1016/j.joen.2017.03.005. - DOI - PubMed
-
- Huang Y.-M., Lin Y.-C., Chen C.-Y., Hsieh Y.-Y., Liaw C.-K., Huang S.-W., Tsuang Y.-H., Chen C.-H., Lin F.-H. Thermosensitive Chitosan–Gelatin–Glycerol Phosphate Hydrogels as Collagenase Carrier for Tendon–Bone Healing in a Rabbit Model. Polymers. 2020;12:436. doi: 10.3390/polym12020436. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

