Pannexins and Connexins: Their Relevance for Oocyte Developmental Competence
- PMID: 34072911
- PMCID: PMC8199496
- DOI: 10.3390/ijms22115918
Pannexins and Connexins: Their Relevance for Oocyte Developmental Competence
Abstract
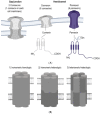
The oocyte is the major determinant of embryo developmental competence in all mammalian species. Although fundamental advances have been generated in the field of reproductive medicine and assisted reproductive technologies in the past three decades, researchers and clinicians are still trying to elucidate molecular factors and pathways, which could be pivotal for the oocyte's developmental competence. The cell-to-cell and cell-to-matrix communications are crucial not only for oocytes but also for multicellular organisms in general. This latter mentioned communication is among others possibly due to the Connexin and Pannexin families of large-pore forming channels. Pannexins belong to a protein group of ATP-release channels, therefore of high importance for the oocyte due to its requirements of high energy supply. An increasing body of studies on Pannexins provided evidence that these channels not only play a role during physiological processes of an oocyte but also during pathological circumstances which could lead to the development of diseases or infertility. Connexins are proteins that form membrane channels and gap-junctions, and more precisely, these proteins enable the exchange of some ions and molecules, and therefore they do play a fundamental role in the communication between the oocyte and accompanying cells. Herein, the role of Pannexins and Connexins for the processes of oogenesis, folliculogenesis, oocyte maturation and fertilization will be discussed and, at the end of this review, Pannexin and Connexin related pathologies and their impact on the developmental competence of oocytes will be provided.
Keywords: connexin; developmental competence; fertilization; maturation; oocyte; oogenesis; pannexin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Pannexin 1 inhibition delays maturation and improves development of Bos taurus oocytes.J Ovarian Res. 2020 Aug 24;13(1):98. doi: 10.1186/s13048-020-00704-w. J Ovarian Res. 2020. PMID: 32838805 Free PMC article.
-
Oocyte-granulosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence.Dev Biol. 2000 Oct 15;226(2):167-79. doi: 10.1006/dbio.2000.9863. Dev Biol. 2000. PMID: 11023678
-
Gap junction gene and protein families: Connexins, innexins, and pannexins.Biochim Biophys Acta Biomembr. 2018 Jan;1860(1):5-8. doi: 10.1016/j.bbamem.2017.05.016. Epub 2017 May 27. Biochim Biophys Acta Biomembr. 2018. PMID: 28559187 Free PMC article. Review.
-
Oogenesis in Women: From Molecular Regulatory Pathways and Maternal Age to Stem Cells.Int J Mol Sci. 2023 Apr 6;24(7):6837. doi: 10.3390/ijms24076837. Int J Mol Sci. 2023. PMID: 37047809 Free PMC article. Review.
-
Phylogenetic and bioinformatic analysis of gap junction-related proteins, innexins, pannexins and connexins.Biomed Res. 2010 Apr;31(2):133-42. doi: 10.2220/biomedres.31.133. Biomed Res. 2010. PMID: 20460741
Cited by
-
Influences of Supplementing Selective Members of the Interleukin-6 Cytokine Family on Bovine Oocyte Competency.Animals (Basel). 2023 Dec 21;14(1):44. doi: 10.3390/ani14010044. Animals (Basel). 2023. PMID: 38200775 Free PMC article.
-
Competencies and contingencies in the expanding ART marketplace: is there a place for precision medicine.J Assist Reprod Genet. 2022 Apr;39(4):779-780. doi: 10.1007/s10815-022-02498-w. J Assist Reprod Genet. 2022. PMID: 35461366 Free PMC article. No abstract available.
-
Age-associated aberrations of the cumulus-oocyte interaction and in the zona pellucida structure reduce fertility in female mice.Commun Biol. 2024 Dec 24;7(1):1692. doi: 10.1038/s42003-024-07305-z. Commun Biol. 2024. PMID: 39719529 Free PMC article.
-
Assessment of Gene Expression on the Gap Junction Connexin of Cumulus Cells on Infertile Women With Polycystic Ovary Syndrome and Poor Ovarian Response: The Novel Role of Propranolol.J Family Reprod Health. 2025 Jun;19(2):111-121. doi: 10.18502/jfrh.v19i2.19299. J Family Reprod Health. 2025. PMID: 40861086 Free PMC article.
-
TRIB3 regulates Cx43 expression in human granulosa cells via the Wnt/β-catenin signaling pathway: an in vitro study.J Ovarian Res. 2025 Jul 2;18(1):139. doi: 10.1186/s13048-025-01694-3. J Ovarian Res. 2025. PMID: 40605006 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

