Effects of Reducing L-Carnitine Supplementation on Carnitine Kinetics and Cardiac Function in Hemodialysis Patients: A Multicenter, Single-Blind, Placebo-Controlled, Randomized Clinical Trial
- PMID: 34073024
- PMCID: PMC8230272
- DOI: 10.3390/nu13061900
Effects of Reducing L-Carnitine Supplementation on Carnitine Kinetics and Cardiac Function in Hemodialysis Patients: A Multicenter, Single-Blind, Placebo-Controlled, Randomized Clinical Trial
Abstract
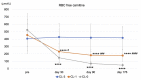
L-carnitine (LC) supplementation improves cardiac function in hemodialysis (HD) patients. However, whether reducing LC supplementation affects carnitine kinetics and cardiac function in HD patients treated with LC remains unclear. Fifty-nine HD patients previously treated with intravenous LC 1000 mg per HD session (three times weekly) were allocated to three groups: LC injection three times weekly, once weekly, and placebo, and prospectively followed up for six months. Carnitine fractions were assessed by enzyme cycling methods. Plasma and red blood cell (RBC) acylcarnitines were profiled using tandem mass spectrometry. Cardiac function was evaluated using echocardiography and plasma B-type natriuretic peptide (BNP) levels. Reducing LC administration to once weekly significantly decreased plasma carnitine fractions and RBC-free carnitine levels during the study period, which were further decreased in the placebo group (p < 0.001). Plasma BNP levels were significantly elevated in the placebo group (p = 0.03). Furthermore, changes in RBC (C16 + C18:1)/C2 acylcarnitine ratio were positively correlated with changes in plasma BNP levels (β = 0.389, p = 0.005). Reducing LC administration for six months significantly decreased both plasma and RBC carnitine levels, while the full termination of LC increased plasma BNP levels; however, it did not influence cardiac function in HD patients.
Keywords: CPT2; acylcarnitine; brain natriuretic peptide; cardiac function; cardiomyopathy; carnitine deficiency; end-stage kidney disease; free fatty acid; heart failure; hemodialysis.
Conflict of interest statement
K.F. has received honoraria, including lecture fees, from Otsuka Pharmaceutical Co., Ltd. All other authors declare no conflict of interest.
Figures



References
-
- House A.A., Wanner C., Sarnak M.J., Piña I.L., McIntyre C.W., Komenda P., Kasiske B.L., Deswal A., de Filippi C.R., Cleland J.G.F., et al. Heart Failure in Chronic Kidney Disease: Conclusions From a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;95:1304–1317. doi: 10.1016/j.kint.2019.02.022. - DOI - PubMed
-
- Nitta K., Abe M., Masakane I., Hanafusa N., Taniguchi M., Hasegawa T., Nakai S., Wada A., Hamano T., Hoshino J., et al. Annual dialysis data report 2018, JSDT Renal Data Registry: Dialysis fluid quality, hemodialysis and hemodiafiltration, peritoneal dialysis, and diabetes. Ren. Replace Ther. 2020;6:51. doi: 10.1186/s41100-020-00290-z. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

