Optimizing External Beam Radiotherapy as per the Risk Group of Localized Prostate Cancer: A Nationwide Multi-Institutional Study (KROG 18-15)
- PMID: 34073100
- PMCID: PMC8198120
- DOI: 10.3390/cancers13112732
Optimizing External Beam Radiotherapy as per the Risk Group of Localized Prostate Cancer: A Nationwide Multi-Institutional Study (KROG 18-15)
Abstract
Purpose: This nationwide multi-institutional study analyzed the patterns of care and outcomes of external beam radiotherapy (EBRT) in localized prostate cancer patients. We compared various risk classification tools and assessed the need for refinements in current radiotherapy (RT) schemes.
Methods and materials: We included non-metastatic prostate cancer patients treated with primary EBRT from 2001 to 2015 in this study. Data of 1573 patients from 17 institutions were analyzed and re-grouped using a risk stratification tool with the highest predictive power for biochemical failure-free survival (BCFFS). We evaluated BCFFS, overall survival (OS), and toxicity rates.
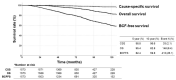
Results: With a median follow-up of 75 months, 5- and 10-year BCFFS rates were 82% and 60%, and 5- and 10-year OS rates were 95% and 83%, respectively. NCCN risk classification revealed the highest predictive power (AUC = 0.556, 95% CI 0.524-0.588; p < 0.001). Gleason score, iPSA < 12 ng/mL, intensity-modulated RT (IMRT), and ≥179 Gy1.5 (EQD2, 77 Gy) were independently significant for BCFFS (all p < 0.05). IMRT and ≥179 Gy1.5 were significant factors in the high-risk group, whereas ≥170 Gy1.5 (EQD2, 72 Gy) was significant in the intermediate-risk group and no significant impact of dose was observed in the low-risk group. Both BCFFS and OS improved significantly when ≥179 Gy1.5 was delivered using IMRT and hypofractionation in the high-risk group without increasing toxicities.
Conclusions: With NCCN risk classification, dose escalation with modern high-precision techniques might increase survivals in the high-risk group, but not in the low-risk group, although mature results of prospective studies are awaited.
Keywords: NCCN; dose-escalation; hypofractionation; prostate cancer; radiotherapy; risk assessment.
Conflict of interest statement
The authors have disclosed that they have not received any financial consideration from any person or organization to support the preparation, analysis, results, or discussion of this article.
Figures




References
-
- D’Amico A.V., Whittington R., Malkowicz S.B., Schultz D., Blank K., Broderick G.A., Tomaszewski J.E., Renshaw A.A., Kaplan I., Beard C.J., et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. - DOI - PubMed
-
- Mohler J.L., Antonarakis E.S., Armstrong A.J., D’Amico A.V., Davis B.J., Dorff T., Eastham J.A., Enke C.A., Farrington T.A., Higano C.S., et al. Prostate cancer, version 2.2019, nccn clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2019;17:479–505. doi: 10.6004/jnccn.2019.0023. - DOI - PubMed
-
- Sanda M.G., Cadeddu J.A., Kirkby E., Chen R.C., Crispino T., Fontanarosa J., Freedland S.J., Greene K., Klotz L.H., Makarov D.V., et al. Clinically localized prostate cancer: Aua/astro/suo guideline. Part i: Risk stratification, shared decision making, and care options. J. Urol. 2018;199:683–690. doi: 10.1016/j.juro.2017.11.095. - DOI - PubMed
LinkOut - more resources
Full Text Sources

