Therapeutic Uses of Bacterial Subunit Toxins
- PMID: 34073185
- PMCID: PMC8226680
- DOI: 10.3390/toxins13060378
Therapeutic Uses of Bacterial Subunit Toxins
Abstract
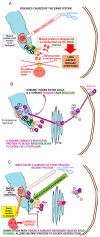
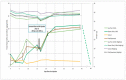
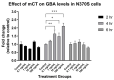
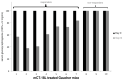
The B subunit pentamer verotoxin (VT aka Shiga toxin-Stx) binding to its cellular glycosphingolipid (GSL) receptor, globotriaosyl ceramide (Gb3) mediates internalization and the subsequent receptor mediated retrograde intracellular traffic of the AB5 subunit holotoxin to the endoplasmic reticulum. Subunit separation and cytosolic A subunit transit via the ER retrotranslocon as a misfolded protein mimic, then inhibits protein synthesis to kill cells, which can cause hemolytic uremic syndrome clinically. This represents one of the most studied systems of prokaryotic hijacking of eukaryotic biology. Similarly, the interaction of cholera AB5 toxin with its GSL receptor, GM1 ganglioside, is the key component of the gastrointestinal pathogenesis of cholera and follows the same retrograde transport pathway for A subunit cytosol access. Although both VT and CT are the cause of major pathology worldwide, the toxin-receptor interaction is itself being manipulated to generate new approaches to control, rather than cause, disease. This arena comprises two areas: anti neoplasia, and protein misfolding diseases. CT/CTB subunit immunomodulatory function and anti-cancer toxin immunoconjugates will not be considered here. In the verotoxin case, it is clear that Gb3 (and VT targeting) is upregulated in many human cancers and that there is a relationship between GSL expression and cancer drug resistance. While both verotoxin and cholera toxin similarly hijack the intracellular ERAD quality control system of nascent protein folding, the more widespread cell expression of GM1 makes cholera the toxin of choice as the means to more widely utilise ERAD targeting to ameliorate genetic diseases of protein misfolding. Gb3 is primarily expressed in human renal tissue. Glomerular endothelial cells are the primary VT target but Gb3 is expressed in other endothelial beds, notably brain endothelial cells which can mediate the encephalopathy primarily associated with VT2-producing E. coli infection. The Gb3 levels can be regulated by cytokines released during EHEC infection, which complicate pathogenesis. Significantly Gb3 is upregulated in the neovasculature of many tumours, irrespective of tumour Gb3 status. Gb3 is markedly increased in pancreatic, ovarian, breast, testicular, renal, astrocytic, gastric, colorectal, cervical, sarcoma and meningeal cancer relative to the normal tissue. VT has been shown to be effective in mouse xenograft models of renal, astrocytoma, ovarian, colorectal, meningioma, and breast cancer. These studies are herein reviewed. Both CT and VT (and several other bacterial toxins) access the cell cytosol via cell surface ->ER transport. Once in the ER they interface with the protein folding homeostatic quality control pathway of the cell -ERAD, (ER associated degradation), which ensures that only correctly folded nascent proteins are allowed to progress to their cellular destinations. Misfolded proteins are translocated through the ER membrane and degraded by cytosolic proteosome. VT and CT A subunits have a C terminal misfolded protein mimic sequence to hijack this transporter to enter the cytosol. This interface between exogenous toxin and genetically encoded endogenous mutant misfolded proteins, provides a new therapeutic basis for the treatment of such genetic diseases, e.g., Cystic fibrosis, Gaucher disease, Krabbe disease, Fabry disease, Tay-Sachs disease and many more. Studies showing the efficacy of this approach in animal models of such diseases are presented.
Keywords: endoplasmic reticulum associated degradation; neoplastic Gb3 expression; protein misfolding diseases; retrograde transport.
Conflict of interest statement
Lingwood is a founder of ERAD Therapeutics (eradtx.cpm) and has received travel support to present the new data reported herein.
Figures
References
-
- Nakao H., Takeda T. Escherichia coli Shiga toxin. J. Nat. Toxins. 2000;9:299–313. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

