Embryonic Trophectoderm Secretomics Reveals Chemotactic Migration and Intercellular Communication of Endometrial and Circulating MSCs in Embryonic Implantation
- PMID: 34073234
- PMCID: PMC8199457
- DOI: 10.3390/ijms22115638
Embryonic Trophectoderm Secretomics Reveals Chemotactic Migration and Intercellular Communication of Endometrial and Circulating MSCs in Embryonic Implantation
Abstract
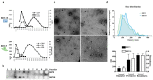
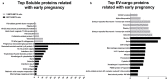
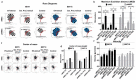
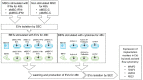
Embryonic implantation is a key step in the establishment of pregnancy. In the present work, we have carried out an in-depth proteomic analysis of the secretome (extracellular vesicles and soluble proteins) of two bovine blastocysts embryonic trophectoderm primary cultures (BBT), confirming different epithelial-mesenchymal transition stages in these cells. BBT-secretomes contain early pregnancy-related proteins and angiogenic proteins both as cargo in EVs and the soluble fraction. We have demonstrated the functional transfer of protein-containing secretome between embryonic trophectoderm and maternal MSC in vitro using two BBT primary cultures eight endometrial MSC (eMSC) and five peripheral blood MSC (pbMSC) lines. We observed that eMSC and pbMSC chemotax to both the soluble fraction and EVs of the BBT secretome. In addition, in a complementary direction, we found that the pattern of expression of implantation proteins in BBT-EVs changes depending on: (i) their epithelial-mesenchymal phenotype; (ii) as a result of the uptake of eMSC- or pbMSC-EV previously stimulated or not with embryonic signals (IFN-); (iii) because of the stimulation with the endometrial cytokines present in the uterine fluid in the peri-implantation period.
Keywords: cell migration; epithelial to mesenchymal transition; extracellular vesicles; mesenchymal stromal cells; trophectoderm.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Mesenchymal Stem Cells in Embryo-Maternal Communication under Healthy Conditions or Viral Infections: Lessons from a Bovine Model.Cells. 2022 Jun 7;11(12):1858. doi: 10.3390/cells11121858. Cells. 2022. PMID: 35740987 Free PMC article. Review.
-
Human Endometrial Extracellular Vesicles Functionally Prepare Human Trophectoderm Model for Implantation: Understanding Bidirectional Maternal-Embryo Communication.Proteomics. 2019 Dec;19(23):e1800423. doi: 10.1002/pmic.201800423. Epub 2019 Oct 30. Proteomics. 2019. PMID: 31531940
-
Bovine peripheral blood MSCs chemotax towards inflammation and embryo implantation stimuli.J Cell Physiol. 2021 Feb;236(2):1054-1067. doi: 10.1002/jcp.29915. Epub 2020 Jul 2. J Cell Physiol. 2021. PMID: 32617972
-
Exosomes and soluble secretome from hormone-treated endometrial epithelial cells direct embryo implantation.Mol Hum Reprod. 2020 Jul 1;26(7):510-520. doi: 10.1093/molehr/gaaa034. Mol Hum Reprod. 2020. PMID: 32402079
-
Emerging Role of Extracellular Vesicles in Embryo-Maternal Communication throughout Implantation Processes.Int J Mol Sci. 2020 Aug 1;21(15):5523. doi: 10.3390/ijms21155523. Int J Mol Sci. 2020. PMID: 32752293 Free PMC article. Review.
Cited by
-
New Roles for EVs, miRNA and lncRNA in Bovine Embryo Implantation.Front Vet Sci. 2022 Jul 15;9:944370. doi: 10.3389/fvets.2022.944370. eCollection 2022. Front Vet Sci. 2022. PMID: 35909679 Free PMC article. Review.
-
IGF-1 promotes trophectoderm cell proliferation of porcine embryos by activating the Wnt/β-catenin pathway.Cell Commun Signal. 2025 Apr 20;23(1):188. doi: 10.1186/s12964-025-02191-2. Cell Commun Signal. 2025. PMID: 40254588 Free PMC article.
-
Optimization of extracellular vesicle isolation and their separation from lipoproteins by size exclusion chromatography.J Extracell Biol. 2023 Jul 8;2(7):e100. doi: 10.1002/jex2.100. eCollection 2023 Jul. J Extracell Biol. 2023. PMID: 38939075 Free PMC article.
-
The effects of cigarette smoking and nicotine on the therapeutic potential of mesenchymal stem cells.Histol Histopathol. 2022 Feb;37(2):93-100. doi: 10.14670/HH-18-400. Epub 2021 Nov 30. Histol Histopathol. 2022. PMID: 34845711 Review.
-
Cathepsin-L Secreted by High-Quality Bovine Embryos Exerts an Embryotrophic Effect In Vitro.Int J Mol Sci. 2023 Mar 31;24(7):6563. doi: 10.3390/ijms24076563. Int J Mol Sci. 2023. PMID: 37047535 Free PMC article.
References
-
- Lee B., Villarreal-Ponce A., Fallahi M., Ovadia J., Sun P., Yu Q.C., Ito S., Sinha S., Nie Q., Dai X. Transcriptional mechanisms link epithelial plasticity to adhesion and differentiation of epidermal progenitor cells. Dev. Cell. 2014;29:47–58. doi: 10.1016/j.devcel.2014.03.005. - DOI - PMC - PubMed

