Multifunctional Scaffolds and Synergistic Strategies in Tissue Engineering and Regenerative Medicine
- PMID: 34073311
- PMCID: PMC8230126
- DOI: 10.3390/pharmaceutics13060792
Multifunctional Scaffolds and Synergistic Strategies in Tissue Engineering and Regenerative Medicine
Abstract
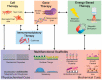
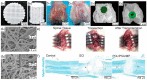
The increasing demand for organ replacements in a growing world with an aging population as well as the loss of tissues and organs due to congenital defects, trauma and diseases has resulted in rapidly evolving new approaches for tissue engineering and regenerative medicine (TERM). The extracellular matrix (ECM) is a crucial component in tissues and organs that surrounds and acts as a physical environment for cells. Thus, ECM has become a model guide for the design and fabrication of scaffolds and biomaterials in TERM. However, the fabrication of a tissue/organ replacement or its regeneration is a very complex process and often requires the combination of several strategies such as the development of scaffolds with multiple functionalities and the simultaneous delivery of growth factors, biochemical signals, cells, genes, immunomodulatory agents, and external stimuli. Although the development of multifunctional scaffolds and biomaterials is one of the most studied approaches for TERM, all these strategies can be combined among them to develop novel synergistic approaches for tissue regeneration. In this review we discuss recent advances in which multifunctional scaffolds alone or combined with other strategies have been employed for TERM purposes.
Keywords: biomaterials; combination therapy; multifunctional materials; scaffolds; tissue engineering and regenerative medicine (TERM).
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

