In Vitro Framework to Assess the Anti- Helicobacter pylori Potential of Lactic Acid Bacteria Secretions as Alternatives to Antibiotics
- PMID: 34073352
- PMCID: PMC8198849
- DOI: 10.3390/ijms22115650
In Vitro Framework to Assess the Anti- Helicobacter pylori Potential of Lactic Acid Bacteria Secretions as Alternatives to Antibiotics
Abstract
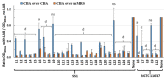
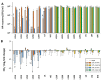
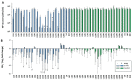
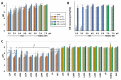
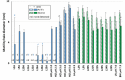
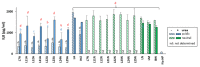
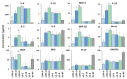
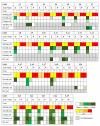
Helicobacter pylori is a prevalent bacterium that can cause gastric ulcers and cancers. Lactic acid bacteria (LAB) ameliorate treatment outcomes against H. pylori, suggesting that they could be a source of bioactive molecules usable as alternatives to current antibiotics for which resistance is mounting. We developed an in vitro framework to compare the anti-H. pylori properties of 25 LAB and their secretions against H. pylori. All studies were done at acidic and neutralized pH, with or without urea to mimic various gastric compartments. Eighteen LAB strains secreted molecules that curtailed the growth of H. pylori and the activity was urea-resistant in five LAB. Several LAB supernatants also reduced the urease activity of H. pylori. Pre-treatment of H. pylori with acidic LAB supernatants abrogated its flagella-mediated motility and decreased its ability to elicit pro-inflammatory IL-8 cytokine from human gastric cells, without reverting the H. pylori-induced repression of other pro-inflammatory cytokines. This study identified the LAB that have the most anti-H. pylori effects, decreasing its viability, its production of virulence factors, its motility and/or its ability to elicit pro-inflammatory IL-8 from gastric cells. Once identified, these molecules can be used as alternatives or complements to current antibiotics to fight H. pylori infections.
Keywords: Helicobacter pylori; cytokines; gastric disease; inflammation; lactic acid bacteria; secretome; urease.
Conflict of interest statement
The authors C.C., S.A.W., M.M.M., S.S. and J.J. declare no conflict of interest. The authors T.A.T. and C.W.M. are LHS employees who participated to this study and LHS funded part of these studies.
Figures
Similar articles
-
Assays for Anti-Helicobacter pylori Effects of Lactic Acid Bacteria Secretions.Methods Mol Biol. 2025;2942:63-79. doi: 10.1007/978-1-0716-4627-4_6. Methods Mol Biol. 2025. PMID: 40498307
-
Antagonistic activity of spent culture supernatants of lactic acid bacteria against Helicobacter pylori growth and infection in human gastric epithelial AGS cells.J Food Sci. 2009 Aug;74(6):M225-30. doi: 10.1111/j.1750-3841.2009.01194.x. J Food Sci. 2009. PMID: 19723205
-
Use of selected lactic acid bacteria in the eradication of Helicobacter pylori infection.J Microbiol. 2014 Nov;52(11):955-62. doi: 10.1007/s12275-014-4355-y. Epub 2014 Oct 3. J Microbiol. 2014. PMID: 25277407
-
Unique mechanism of Helicobacter pylori for colonizing the gastric mucus.Microbes Infect. 2000 Jan;2(1):55-60. doi: 10.1016/s1286-4579(00)00285-9. Microbes Infect. 2000. PMID: 10717541 Review.
-
Probiotics and Helicobacter pylori.Best Pract Res Clin Gastroenterol. 2003 Oct;17(5):785-91. doi: 10.1016/s1521-6918(03)00070-2. Best Pract Res Clin Gastroenterol. 2003. PMID: 14507588 Review.
Cited by
-
The role of gastric microecological dysbiosis in gastric carcinogenesis.Front Microbiol. 2023 Jul 31;14:1218395. doi: 10.3389/fmicb.2023.1218395. eCollection 2023. Front Microbiol. 2023. PMID: 37583514 Free PMC article. Review.
-
Microbiota and the Immune System-Actors in the Gastric Cancer Story.Cancers (Basel). 2022 Aug 8;14(15):3832. doi: 10.3390/cancers14153832. Cancers (Basel). 2022. PMID: 35954495 Free PMC article. Review.
-
The Influence of Gastric Microbiota and Probiotics in Helicobacter pylori Infection and Associated Diseases.Biomedicines. 2024 Dec 30;13(1):61. doi: 10.3390/biomedicines13010061. Biomedicines. 2024. PMID: 39857645 Free PMC article. Review.
-
The impacts of probiotics in eradication therapy of Helicobacter pylori.Arch Microbiol. 2022 Nov 7;204(12):692. doi: 10.1007/s00203-022-03314-w. Arch Microbiol. 2022. PMID: 36344628 Free PMC article. Review.
-
A review for non-antibiotic treatment of Helicobacter pylori: new insight.Front Microbiol. 2024 May 7;15:1379209. doi: 10.3389/fmicb.2024.1379209. eCollection 2024. Front Microbiol. 2024. PMID: 38774508 Free PMC article. Review.
References
-
- Malfertheiner P., Sipponen P., Naumann M., Moayyedi P., Mégraud F., Xiao S.-D., Sugano K., Nyrén O., Force T.L.H.P.-G.C.T. Helicobacter pylori Eradication Has the Potential to Prevent Gastric Cancer: A State-of-the-Art Critique. Am. J. Gastroenterol. 2005;100:2100–2115. doi: 10.1111/j.1572-0241.2005.41688.x. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

