The Effect of Dietary Fibre on Gut Microbiota, Lipid Profile, and Inflammatory Markers in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials
- PMID: 34073366
- PMCID: PMC8228854
- DOI: 10.3390/nu13061805
The Effect of Dietary Fibre on Gut Microbiota, Lipid Profile, and Inflammatory Markers in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials
Abstract
Background: A disequilibrium of the gut microbial community has been closely associated with systemic inflammation and metabolic syndromes including type 2 diabetes. While low fibre and high fat diets may lead to dysbiosis of the gut microbiome as a result of the loss of useful microbes, it has been reported that a high fibre diet may prevent the fermentation of protein and may promote eubiosis of gut microbiota.
Aim: This review aims to evaluate the effect of dietary fibre (DF) on gut microbiota, lipid profile, and inflammatory markers in patients with type 2 diabetes.
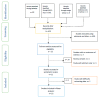
Methods: The PRISMA framework was relied on to conduct this systematic review and meta-analysis. Searches were carried out using electronic databases and reference list of articles.
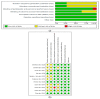
Results: Eleven studies were included in the systematic review, while ten studies were included in the meta-analysis. The findings revealed five distinct areas including the effects of DF on (a) gut microbiota (122 participants); (b) lipopolysaccharides (LPS, 79 participants) and lipopolysaccharides binding protein (LBP, 81 participants); (c) lipid profile; (d) inflammatory markers; and (e) body mass index (BMI, 319 participants). The relative abundance of Bifidobacterium increased by 0.73 (95% CI: 0.57, 0.89) in the DF group in contrast to the control (p < 0.05). With respect to LPS, the level was lower in the DF group than the control and the difference was significant (p < 0.05). The standardised mean difference for LPS was -0.45 (95% CI: -0.90, -0.01) although the difference between the two groups in relation to LBP was not significant (p = 0.08) and the mean difference was 0.92 (95% CI: -0.12, 1.95). While there was a decrease of -1.05 (95% CI: -2.07, -0.02) with respect to total cholesterol (356 participants) in the DF group as compared with the control (p < 0.05), both groups were not significantly different (p > 0.05) in the other lipid parameters. The difference between the groups was significant (p < 0.05) in relation to C-reactive protein, and the mean difference was 0.43 (95% CI: 0.02, 0.84). This could be due to the short duration of the included studies and differences in participants' diets including the amount of dietary fibre supplements. However, the groups were not significantly different (p > 0.05) with respect to the other inflammatory markers. The meta-analysis of the BMI showed that the DF group decreased by -0.57 (95% CI: -1.02, -0.12) as compared with the control and this was significant (p < 0.01).
Conclusion: DF significantly (p < 0.05) increased the relative abundance of Bifidobacterium and significantly decreased (p < 0.05) LPS, total cholesterol, and BMI as compared with the control. However, DF did not seem to have an effect that was significant on LBP, triglyceride, HDL cholesterol, LDL cholesterol, IL-6, TNF-α, adiponectin, and leptin. These findings have implications for public health in relation to the use of dietary fibre in nutritional interventions and as strategies for managing type 2 diabetes.
Keywords: body mass index; dietary fibre; gut microbiota; inflammatory markers; lipid profile; lipopolysaccharide; type 2 diabetes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
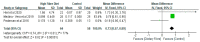






Similar articles
-
The Role of Dietary Fibre in Modulating Gut Microbiota Dysbiosis in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials.Nutrients. 2020 Oct 23;12(11):3239. doi: 10.3390/nu12113239. Nutrients. 2020. PMID: 33113929 Free PMC article.
-
The Effects of Almonds on Gut Microbiota, Glycometabolism, and Inflammatory Markers in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials.Nutrients. 2021 Sep 26;13(10):3377. doi: 10.3390/nu13103377. Nutrients. 2021. PMID: 34684378 Free PMC article.
-
Dietary intake of fat and fibre according to reference values relates to higher gut microbiota richness in overweight pregnant women.Br J Nutr. 2017 Sep;118(5):343-352. doi: 10.1017/S0007114517002100. Br J Nutr. 2017. PMID: 28901891
-
Effects of defatted rice bran-fortified bread on gut microbiome, cardiovascular risk, gut discomfort, wellbeing and gut physiology in healthy adults with low dietary fibre intake.Clin Nutr ESPEN. 2025 Jun;67:362-376. doi: 10.1016/j.clnesp.2025.03.045. Epub 2025 Mar 22. Clin Nutr ESPEN. 2025. PMID: 40127766 Clinical Trial.
-
The Effects of a Low GI Diet on Cardiometabolic and Inflammatory Parameters in Patients with Type 2 and Gestational Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials.Nutrients. 2019 Jul 12;11(7):1584. doi: 10.3390/nu11071584. Nutrients. 2019. PMID: 31336986 Free PMC article.
Cited by
-
The effect of different dietary restriction on weight management and metabolic parameters in people with type 2 diabetes mellitus: a network meta-analysis of randomized controlled trials.Diabetol Metab Syndr. 2024 Oct 28;16(1):254. doi: 10.1186/s13098-024-01492-9. Diabetol Metab Syndr. 2024. PMID: 39468618 Free PMC article. Review.
-
Variations in the Relative Abundance of Gut Bacteria Correlate with Lipid Profiles in Healthy Adults.Microorganisms. 2023 Oct 28;11(11):2656. doi: 10.3390/microorganisms11112656. Microorganisms. 2023. PMID: 38004667 Free PMC article.
-
Impact of Dietary Fiber on Inflammation in Humans.Int J Mol Sci. 2025 Feb 25;26(5):2000. doi: 10.3390/ijms26052000. Int J Mol Sci. 2025. PMID: 40076626 Free PMC article. Review.
-
Association between seaweed intake and risk of type 2 diabetes mellitus: a prospective cohort study.Br J Nutr. 2024 Apr 14;131(7):1259-1267. doi: 10.1017/S0007114523002751. Epub 2023 Nov 28. Br J Nutr. 2024. PMID: 38012847 Free PMC article.
-
Effects of an inulin fiber diet on the gut microbiome, colon, and inflammatory biomarkers in aged mice.Exp Gerontol. 2023 Jun 1;176:112164. doi: 10.1016/j.exger.2023.112164. Epub 2023 Apr 7. Exp Gerontol. 2023. PMID: 37011713 Free PMC article.
References
-
- Carrera-Quintanar L., López Roa R.I., Quintero-Fabián S., Sánchez-Sánchez M.A., Vizmanos B., Ortuño-Sahagún D. Phytochemicals that influence gut microbiota as prophylactics and for the treatment of obesity and inflammatory diseases. Mediat. Inflamm. 2018;2018:9734845. doi: 10.1155/2018/9734845. - DOI - PMC - PubMed
-
- Aliasgharzadeh A., Aliloo A., Ghotaslou R., Arbabi S. Comparison of bifidobacterium spp. and lactobacillus spp. count in faeces of patients with type 2 diabetes mellitus and healthy people. Middle East J. Fam. Med. 2018;16:102–106. doi: 10.5742/MEWFM.2018.93316. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

