Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon
- PMID: 34073401
- PMCID: PMC8226630
- DOI: 10.3390/v13060989
Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon
Retraction in
-
Retraction: Samaha et al. Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon. Viruses 2021, 13, 989.Viruses. 2021 Oct 26;13(11):2154. doi: 10.3390/v13112154. Viruses. 2021. PMID: 34751680 Free PMC article.
Abstract
Objective: This study was designed to determine the efficacy of ivermectin, an FDA-approved drug, in producing clinical benefits and decreasing the viral load of SARS-CoV-2 among asymptomatic subjects that tested positive for this virus in Lebanon.
Methods: A randomized controlled trial was conducted in 100 asymptomatic Lebanese subjects that have tested positive for SARS-CoV2. Fifty patients received standard preventive treatment, mainly supplements, and the experimental group received a single dose (according to body weight) of ivermectin, in addition to the same supplements the control group received.
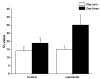
Results: There was no significant difference (p = 0.06) between Ct-values of the two groups before the regimen was started (day zero), indicating that subjects in both groups had similar viral loads. At 72 h after the regimen started, the increase in Ct-values was dramatically higher in the ivermectin than in the control group. In the ivermectin group, Ct increased from 15.13 ± 2.07 (day zero) to 30.14 ± 6.22 (day three; mean ± SD), compared to the control group, where the Ct values increased only from 14.20 ± 2.48 (day zero) to 18.96 ± 3.26 (day three; mean ± SD). Moreover, more subjects in the control group developed clinical symptoms. Three individuals (6%) required hospitalization, compared to the ivermectin group (0%).
Conclusion: Ivermectin appears to be efficacious in providing clinical benefits in a randomized treatment of asymptomatic SARS-CoV-2-positive subjects, effectively resulting in fewer symptoms, lower viral load and reduced hospital admissions. However, larger-scale trials are warranted for this conclusion to be further cemented.
Keywords: COVID-19; Lebanon; SARS-CoV-2; clinical trial; ivermectin; pandemic; therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Lorenz C., Dias Bocewicz A.C., Correa de Azevedo Marques C., Reis Santana L.M., Chiaravalloti-Neto F., Alves Gomes A.H., Barbosa G.L. Have measures against COVID-19 helped to reduce dengue cases in Brazil? Travel. Med. Infect. Dis. 2020;37:101827. doi: 10.1016/j.tmaid.2020.101827. - DOI - PMC - PubMed
-
- Shakkour Z., Habashy K.J., Berro M., Takkoush S., Abdelhady S., Koleilat N., Eid A.H., Zibara K., Obeid M., Shear D., et al. Drug Repurposing in Neurological Disorders: Implications for Neurotherapy in Traumatic Brain Injury. Neuroscientist. 2020:1073858420961078. doi: 10.1177/1073858420961078. - DOI - PubMed
-
- Hammoud S.H., Wehbe Z., Abdelhady S., Kobeissy F., Eid A.H., El-Yazbi A.F. Dysregulation of Angiotensin Converting Enzyme 2 Expression and Function in Comorbid Disease Conditions Possibly Contributes to Coronavirus Infectious Disease 2019 Complication Severity. Mol. Pharmacol. 2021;99:17–28. doi: 10.1124/molpharm.120.000119. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

