Digital Technology in Clinical Trials for Multiple Sclerosis: Systematic Review
- PMID: 34073464
- PMCID: PMC8199078
- DOI: 10.3390/jcm10112328
Digital Technology in Clinical Trials for Multiple Sclerosis: Systematic Review
Abstract
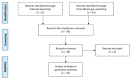
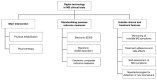
Clinical trials in multiple sclerosis (MS) have been including digital technology tools to overcome limitations in treatment delivery and disease monitoring. In March 2020, we conducted a systematic search on pubmed.gov and clinicaltrials.gov databases (with no restrictions) to identify all relevant published and unpublished clinical trials, in English language, including MS patients, in which digital technology was applied. We used "multiple sclerosis" and "clinical trial" as the main search words, and "app", "digital", "electronic", "internet" and "mobile" as additional search words, separately. Digital technology is part of clinical trial interventions to deliver psychotherapy and motor rehabilitation, with exergames, e-training, and robot-assisted exercises. Digital technology has been used to standardise previously existing outcome measures, with automatic acquisitions, reduced inconsistencies, and improved detection of symptoms (e.g., electronic recording of motor performance). Other clinical trials have been using digital technology for monitoring symptoms that would be otherwise difficult to detect (e.g., fatigue, balance), for measuring treatment adherence and side effects, and for self-assessment purposes. Collection of outcome measures is progressively shifting from paper-based on site, to internet-based on site, and, in the future, to internet-based at home, with the detection of clinical and treatment features that would have remained otherwise invisible. Similarly, remote interventions provide new possibilities of motor and cognitive rehabilitation.
Keywords: clinical trial; digital technology; multiple sclerosis; outcome measures.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources