Proteomic Comparison of Bone Marrow Derived Osteoblasts and Mesenchymal Stem Cells
- PMID: 34073480
- PMCID: PMC8198503
- DOI: 10.3390/ijms22115665
Proteomic Comparison of Bone Marrow Derived Osteoblasts and Mesenchymal Stem Cells
Abstract
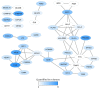
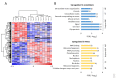
Mesenchymal stem cells (MSCs) can differentiate into osteoblasts, and therapeutic targeting of these cells is considered both for malignant and non-malignant diseases. We analyzed global proteomic profiles for osteoblasts derived from ten and MSCs from six healthy individuals, and we quantified 5465 proteins for the osteoblasts and 5420 proteins for the MSCs. There was a large overlap in the profiles for the two cell types; 156 proteins were quantified only in osteoblasts and 111 proteins only for the MSCs. The osteoblast-specific proteins included several extracellular matrix proteins and a network including 27 proteins that influence intracellular signaling (Wnt/Notch/Bone morphogenic protein pathways) and bone mineralization. The osteoblasts and MSCs showed only minor age- and sex-dependent proteomic differences. Finally, the osteoblast and MSC proteomic profiles were altered by ex vivo culture in serum-free media. We conclude that although the proteomic profiles of osteoblasts and MSCs show many similarities, we identified several osteoblast-specific extracellular matrix proteins and an osteoblast-specific intracellular signaling network. Therapeutic targeting of these proteins will possibly have minor effects on MSCs. Furthermore, the use of ex vivo cultured osteoblasts/MSCs in clinical medicine will require careful standardization of the ex vivo handling of the cells.
Keywords: bone marrow; ex vivo handling; in vitro culture; mesenchymal stem cell; osteoblast; proteome.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts.J Bone Miner Res. 2007 Dec;22(12):1943-56. doi: 10.1359/jbmr.070725. J Bone Miner Res. 2007. PMID: 17680726
-
atf4 promotes β-catenin expression and osteoblastic differentiation of bone marrow mesenchymal stem cells.Int J Biol Sci. 2013;9(3):256-66. doi: 10.7150/ijbs.5898. Epub 2013 Feb 27. Int J Biol Sci. 2013. PMID: 23494915 Free PMC article.
-
Differential expression of cell cycle and WNT pathway-related genes accounts for differences in the growth and differentiation potential of Wharton's jelly and bone marrow-derived mesenchymal stem cells.Stem Cell Res Ther. 2017 Apr 26;8(1):102. doi: 10.1186/s13287-017-0555-9. Stem Cell Res Ther. 2017. PMID: 28446235 Free PMC article.
-
PPARγ and Wnt Signaling in Adipogenic and Osteogenic Differentiation of Mesenchymal Stem Cells.Curr Stem Cell Res Ther. 2016;11(3):216-25. doi: 10.2174/1574888x10666150519093429. Curr Stem Cell Res Ther. 2016. PMID: 25986621 Review.
-
Osteogenic differentiation of human marrow-derived mesenchymal stem cells.Regen Med. 2006 Jul;1(4):539-48. doi: 10.2217/17460751.1.4.539. Regen Med. 2006. PMID: 17465848 Review.
Cited by
-
Proteomic Biomarkers Associated with Low Bone Mineral Density: A Systematic Review.Int J Mol Sci. 2024 Jul 9;25(14):7526. doi: 10.3390/ijms25147526. Int J Mol Sci. 2024. PMID: 39062769 Free PMC article.
-
Mass Spectrometry-Based Proteomics Workflows in Cancer Research: The Relevance of Choosing the Right Steps.Cancers (Basel). 2023 Jan 16;15(2):555. doi: 10.3390/cancers15020555. Cancers (Basel). 2023. PMID: 36672506 Free PMC article. Review.
-
Functionalizing Collagen Membranes with MSC-Conditioned Media Promotes Guided Bone Regeneration in Rat Calvarial Defects.Cells. 2023 Feb 28;12(5):767. doi: 10.3390/cells12050767. Cells. 2023. PMID: 36899904 Free PMC article.
-
Molecular Morphology and Function of Stromal Cells.Int J Mol Sci. 2021 Dec 14;22(24):13422. doi: 10.3390/ijms222413422. Int J Mol Sci. 2021. PMID: 34948214 Free PMC article.
-
Proteomic Analysis of Mesenchymal Stromal Cells Secretome in Comparison to Leukocyte- and Platelet-Rich Fibrin.Int J Mol Sci. 2023 Aug 22;24(17):13057. doi: 10.3390/ijms241713057. Int J Mol Sci. 2023. PMID: 37685865 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

