Therapeutics Targeting the Core Apoptotic Machinery
- PMID: 34073507
- PMCID: PMC8198123
- DOI: 10.3390/cancers13112618
Therapeutics Targeting the Core Apoptotic Machinery
Erratum in
-
Correction: Hamilton et al. Therapeutics Targeting the Core Apoptotic Machinery. Cancers 2021, 13, 2618.Cancers (Basel). 2022 Mar 11;14(6):1441. doi: 10.3390/cancers14061441. Cancers (Basel). 2022. PMID: 35326748 Free PMC article.
Abstract
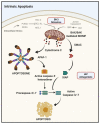
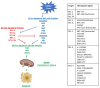
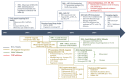
Therapeutic targeting of the apoptotic pathways for the treatment of cancer is emerging as a valid and exciting approach in anti-cancer therapeutics. Accumulating evidence demonstrates that cancer cells are typically "addicted" to a small number of anti-apoptotic proteins for their survival, and direct targeting of these proteins could provide valuable approaches for directly killing cancer cells. Several approaches and agents are in clinical development targeting either the intrinsic mitochondrial apoptotic pathway or the extrinsic death receptor mediated pathways. In this review, we discuss the main apoptosis pathways and the key molecular targets which are the subject of several drug development approaches, the clinical development of these agents and the emerging resistance factors and combinatorial treatment approaches for this class of agents with existing and emerging novel targeted anti-cancer therapeutics.
Keywords: FLIP; apoptosis; cancer therapeutics; resistance.
Conflict of interest statement
The authors declare no conflict of interest and funders had no role in the design of the review; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Galluzzi L., Vitale I., Aaronson S.A., Abrams J.M., Adam D., Agostinis P., Alnemri E.S., Altucci L., Amelio I., Andrews D.W., et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

