COVID-19 Vaccine and Death: Causality Algorithm According to the WHO Eligibility Diagnosis
- PMID: 34073536
- PMCID: PMC8229116
- DOI: 10.3390/diagnostics11060955
COVID-19 Vaccine and Death: Causality Algorithm According to the WHO Eligibility Diagnosis
Abstract
The current challenge worldwide is the administration of anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines. Even if rarely, severe vascular adverse reactions temporally related to vaccine administration have induced diffidence in the population at large. In particular, researchers worldwide are focusing on the so-called "thrombosis and thrombocytopenia after COVID-19 vaccination". This study aims to establish a practical workflow to define the relationship between adverse events following immunization (AEFI) and COVID-19 vaccination, following the basic framework of the World Health Organization (WHO). Post-mortem investigation plays a pivotal role to support this causality relationship when death occurs. To demonstrate the usefulness and feasibility of the proposed workflow, we applied it to two exemplificative cases of suspected AEFI following COVID-19 vaccination. Based on the proposed model, we took into consideration any possible causality relationship between COVID-19 vaccine administration and AEFI. This led us to conclude that vaccination with ChAdOx1 nCov-19 may cause the rare development of immune thrombocytopenia mediated by platelet-activating antibodies against platelet factor 4 (PF4), which clinically mimics heparin-induced autoimmune thrombocytopenia. We suggest the adoption of the proposed methodology in order to confirm or rule out a causal relationship between vaccination and the occurrence of AEFI.
Keywords: COVID-19; SARS-CoV-2; autopsy; deep vein thrombosis; disseminated intravascular coagulation; immune thrombocytopenia; post-mortem investigation; standard protocol; vaccination campaign; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
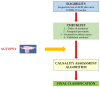
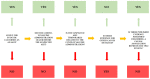

References
-
- EMA EMA Recommends First COVID-19 Vaccine for Authorisation in the EU. [(accessed on 27 March 2021)]; Available online: https://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-....
-
- EMA EMA Recommends COVID-19 Vaccine Moderna for Authorisation in the EU. [(accessed on 27 March 2021)]; Available online: https://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-modern....
-
- EMA EMA Recommends COVID-19 Vaccine AstraZeneca for Authorisation in the EU. [(accessed on 27 March 2021)]; Available online: https://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-astraz....
-
- EMA EMA Recommends COVID-19 Vaccine Janssen for Authorisation in the EU. [(accessed on 13 April 2021)]; Available online: https://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-jansse....
LinkOut - more resources
Full Text Sources
Miscellaneous

