The C0-C1f Region of Cardiac Myosin Binding Protein-C Induces Pro-Inflammatory Responses in Fibroblasts via TLR4 Signaling
- PMID: 34073556
- PMCID: PMC8230336
- DOI: 10.3390/cells10061326
The C0-C1f Region of Cardiac Myosin Binding Protein-C Induces Pro-Inflammatory Responses in Fibroblasts via TLR4 Signaling
Abstract
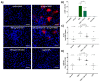
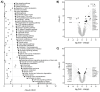
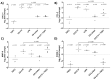
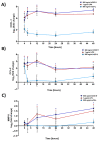
Myocardial injury is associated with inflammation and fibrosis. Cardiac myosin-binding protein-C (cMyBP-C) is cleaved by µ-calpain upon myocardial injury, releasing C0-C1f, an N-terminal peptide of cMyBP-C. Previously, we reported that the presence of C0-C1f is pathogenic within cardiac tissue and is able to activate macrophages. Fibroblasts also play a crucial role in cardiac remodeling arising from ischemic events, as they contribute to both inflammation and scar formation. To understand whether C0-C1f directly modulates fibroblast phenotype, we analyzed the impact of C0-C1f on a human fibroblast cell line in vitro by performing mRNA microarray screening, immunofluorescence staining, and quantitative real-time PCR. The underlying signaling pathways were investigated by KEGG analysis and determined more precisely by targeted inhibition of the potential signaling cascades in vitro. C0-C1f induced pro-inflammatory responses that might delay TGFβ-mediated myofibroblast conversion. TGFβ also counteracted C0-C1f-mediated fibroblast activation. Inhibition of TLR4 or NFκB as well as the delivery of miR-146 significantly reduced C0-C1f-mediated effects. In conclusion, C0-C1f induces inflammatory responses in human fibroblasts that are mediated via TRL4 signaling, which is decreased in the presence of TGFβ. Specific targeting of TLR4 signaling could be an innovative strategy to modulate C0-C1f-mediated inflammation.
Keywords: C0-C1f; MYBPC3; cMyBP-C; fibroblasts; inflammation; miRNA-146.
Conflict of interest statement
Sadayappan provided consulting and collaborative research studies to the Leducq Foundation, Red Saree Inc., Greater Cincinnati Tamil Sangam, AstraZeneca, MyoKardia, Merck and Amgen, but such work is unrelated to the content of this manuscript. No other disclosures are reported. Truschel is an employee of InSCREENeX GmbH, which commercializes the immortalized cell line (huFib) described in this work.
Figures
References
-
- Mouton A.J., DeLeon-Pennell K.Y., Rivera Gonzalez O.J., Flynn E.R., Freeman T.C., Saucerman J.J., Garrett M.R., Ma Y., Harmancey R., Lindsey M.L. Mapping macrophage polarization over the myocardial infarction time continuum. Basic Res. Cardiol. 2018;113:26. doi: 10.1007/s00395-018-0686-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

