The Multifaceted Nature of Nucleobindin-2 in Carcinogenesis
- PMID: 34073612
- PMCID: PMC8198689
- DOI: 10.3390/ijms22115687
The Multifaceted Nature of Nucleobindin-2 in Carcinogenesis
Abstract
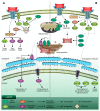
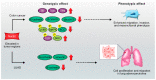
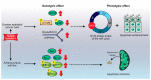
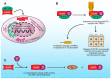
Nucb2 is a multifunctional protein associated with a variety of biological processes. Multiple studies have revealed that Nucb2, and its derivative nesfatin-1, are involved in carcinogenesis. Interestingly, the role of Nucb2/nesfatin-1 in tumorigenesis seems to be dual-both pro-metastatic and anti-metastatic. The implication of Nucb2/nesfatin-1 in carcinogenesis seems to be tissue dependent. Herein, we review the role of Nucb2/nesfatin-1 in both carcinogenesis and the apoptosis process, and we also highlight the multifaceted nature of Nucb2/nesfatin-1.
Keywords: Nucb2; apoptosis; biomarker; carcinogenesis; nesfatin-1; tumors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Nucleobindin-2/Nesfatin-1-A New Cancer Related Molecule?Int J Mol Sci. 2021 Aug 2;22(15):8313. doi: 10.3390/ijms22158313. Int J Mol Sci. 2021. PMID: 34361082 Free PMC article. Review.
-
Nucleobindin-2/Nesfatin-1 in the Human Hypothalamus Is Reduced in Obese Subjects and Colocalizes with Oxytocin, Vasopressin, Melanin-Concentrating Hormone, and Cocaine- and Amphetamine-Regulated Transcript.Neuroendocrinology. 2019;108(3):190-200. doi: 10.1159/000496731. Epub 2019 Jan 9. Neuroendocrinology. 2019. PMID: 30625474
-
NUCB2/Nesfatin-1 drives breast cancer metastasis through the up-regulation of cholesterol synthesis via the mTORC1 pathway.J Transl Med. 2023 Jun 5;21(1):362. doi: 10.1186/s12967-023-04236-x. J Transl Med. 2023. PMID: 37277807 Free PMC article.
-
Loss of Nucleobindin-2/Nesfatin-1 increases lipopolysaccharide-induced murine acute lung inflammation.Cell Tissue Res. 2021 Jul;385(1):87-103. doi: 10.1007/s00441-021-03435-6. Epub 2021 Mar 30. Cell Tissue Res. 2021. PMID: 33783610
-
Effects of physical activity and exercise on Nucleobindin-2 gene expression and Nesfatin-1 concentration: A rapid review.Cell Biochem Funct. 2023 Dec;41(8):1016-1030. doi: 10.1002/cbf.3877. Epub 2023 Nov 1. Cell Biochem Funct. 2023. PMID: 37909689 Review.
Cited by
-
Nesfatin-3 possesses divalent metal ion binding properties, which remain hidden in the nucleobindin-2 precursor protein.Cell Commun Signal. 2023 Jun 29;21(1):165. doi: 10.1186/s12964-023-01181-6. Cell Commun Signal. 2023. PMID: 37386441 Free PMC article.
-
LINC00511 promotes melanoma progression by targeting miR-610/NUCB2.Open Med (Wars). 2023 Mar 1;18(1):20230628. doi: 10.1515/med-2023-0628. eCollection 2023. Open Med (Wars). 2023. PMID: 36874361 Free PMC article.
-
The dark side of SIRT7.Mol Cell Biochem. 2024 Nov;479(11):2843-2861. doi: 10.1007/s11010-023-04869-y. Epub 2023 Dec 8. Mol Cell Biochem. 2024. PMID: 38064138 Review.
-
The effect of periodontal health and disease on interleukin 1β and nesfatin-1 levels in gingival crevicular fluid: A cross-sectional study.Cent Eur J Immunol. 2024;49(2):187-193. doi: 10.5114/ceji.2024.140637. Epub 2024 Jul 10. Cent Eur J Immunol. 2024. PMID: 39381549 Free PMC article.
-
Nucleobindin 2 inhibits senescence in gastric carcinoma.Sci Rep. 2024 May 17;14(1):11261. doi: 10.1038/s41598-024-61111-5. Sci Rep. 2024. PMID: 38760405 Free PMC article.
References
-
- Barnikol-Watanabe S., Groß N.A., Götz H., Henkel T., Karabinos A., Kratzin H., Barnikol H.U., Hilschmann N. Human Protein NEFA, a Novel DNA Binding/EF-Hand/Leucine Zipper Protein. Molecular Cloning and Sequence Analysis of the cDNA, Isolation and Characterization of the Protein. Biol. Chem. Hoppe Seyler. 1994;375:497–512. doi: 10.1515/bchm3.1994.375.8.497. - DOI - PubMed
-
- Ramanjaneya M., Chen J., Brown J.E., Tripathi G., Hallschmid M., Patel S., Kern W., Hillhouse E.W., Lehnert H., Tan B.K., et al. Identification of Nesfatin-1 in Human and Murine Adipose Tissue: A Novel Depot-Specific Adipokine with Increased Levels in Obesity. Endocrinology. 2010;151:3169–3180. doi: 10.1210/en.2009-1358. - DOI - PubMed
-
- García-Galiano D., Pineda R., Ilhan T., Castellano J.M., Ruiz-Pino F., Sánchez-Garrido M.A., Vazquez M.J., Sangiao-Alvarellos S., Romero-Ruiz A., Pinilla L., et al. Cellular Distribution, Regulated Expression, and Functional Role of the Anorexigenic Peptide, NUCB2/Nesfatin-1, in the Testis. Endocrinology. 2012;153:1959–1971. doi: 10.1210/en.2011-2032. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

