Fanconi Syndrome Leading to Hypophosphatemic Osteomalacia Related to Tenofovir Use
- PMID: 34073672
- PMCID: PMC8162330
- DOI: 10.3390/idr13020044
Fanconi Syndrome Leading to Hypophosphatemic Osteomalacia Related to Tenofovir Use
Abstract
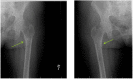
Tenofovir disoproxil fumarate (TDF) is used worldwide to treat and prevent Human Immunodeficiency Virus (HIV) infection. Fanconi syndrome is a complication of TDF use and is characterized by inadequate reabsorption of glucose, phosphate and protein in the proximal tubule of the kidney which may eventually lead to osteomalacia manifested by symptoms of pain, muscular weakness and difficulty ambulating. We present a patient with severe osteomalacia due to progressive and unrecognized Fanconi's syndrome, who responded rapidly to TDF withdrawal, oral phosphate repletion and calcitriol. With the widespread use of TDF-containing antiviral regimens, it is critically important that physicians adhere to screening recommendations to detect early Fanconi syndrome, and recognize symptoms of osteomalacia as a serious complication.
Keywords: Fanconi syndrome; HIV; adverse event; hypophosphatemic osteomalacia; tenofovir.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ending the Aids Epide Micfact Sheet–World Aids Day 2020. [(accessed on 1 December 2020)]; Available online: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_e....
-
- Updated Recommendations on First-Line And Second-Line Antiretroviral Regimens and Post-Exposure Prophylaxis and Recommendations On Early Infant Diagnosis Of Hiv: Interim Guidance. [(accessed on 1 January 2018)]; Available online: http://www.who.int/hiv/pub/guidelines/ARV2018update/en/
-
- Sax P.E., Wohl D., Yin M.T., Post F., DeJesus E., Saag M., Pozniak A., Thompson M., Podzamczer D., Molina J.M., et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: Two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385:2606–2615. doi: 10.1016/S0140-6736(15)60616-X. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

