Understanding the Intricate Web of Phytohormone Signalling in Modulating Root System Architecture
- PMID: 34073675
- PMCID: PMC8197090
- DOI: 10.3390/ijms22115508
Understanding the Intricate Web of Phytohormone Signalling in Modulating Root System Architecture
Abstract
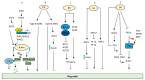
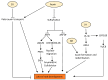
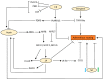
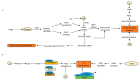
Root system architecture (RSA) is an important developmental and agronomic trait that is regulated by various physical factors such as nutrients, water, microbes, gravity, and soil compaction as well as hormone-mediated pathways. Phytohormones act as internal mediators between soil and RSA to influence various events of root development, starting from organogenesis to the formation of higher order lateral roots (LRs) through diverse mechanisms. Apart from interaction with the external cues, root development also relies on the complex web of interaction among phytohormones to exhibit synergistic or antagonistic effects to improve crop performance. However, there are considerable gaps in understanding the interaction of these hormonal networks during various aspects of root development. In this review, we elucidate the role of different hormones to modulate a common phenotypic output, such as RSA in Arabidopsis and crop plants, and discuss future perspectives to channel vast information on root development to modulate RSA components.
Keywords: phytohormone signalling; root development; root meristem; root system architecture; root system plasticity; root tropic responses.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Hidden branches: developments in root system architecture.Annu Rev Plant Biol. 2007;58:93-113. doi: 10.1146/annurev.arplant.58.032806.104006. Annu Rev Plant Biol. 2007. PMID: 17177637 Review.
-
Light at the end of the tunnel: integrating signaling pathways in the coordination of lateral root development.Biochem Soc Trans. 2024 Aug 28;52(4):1895-1908. doi: 10.1042/BST20240049. Biochem Soc Trans. 2024. PMID: 39171690 Review.
-
Patterning the primary root in Arabidopsis.Wiley Interdiscip Rev Dev Biol. 2012 Sep-Oct;1(5):675-91. doi: 10.1002/wdev.49. Epub 2012 Mar 22. Wiley Interdiscip Rev Dev Biol. 2012. PMID: 23799568 Review.
-
The Xerobranching Response Represses Lateral Root Formation When Roots Are Not in Contact with Water.Curr Biol. 2018 Oct 8;28(19):3165-3173.e5. doi: 10.1016/j.cub.2018.07.074. Epub 2018 Sep 27. Curr Biol. 2018. PMID: 30270188
-
Intrinsic and environmental response pathways that regulate root system architecture.Plant Cell Environ. 2005 Jan;28(1):67-77. doi: 10.1111/j.1365-3040.2005.01306.x. Plant Cell Environ. 2005. PMID: 16021787 Review.
Cited by
-
CRISPR-Based Genome Editing for Nutrient Enrichment in Crops: A Promising Approach Toward Global Food Security.Front Genet. 2022 Jul 14;13:932859. doi: 10.3389/fgene.2022.932859. eCollection 2022. Front Genet. 2022. PMID: 35910203 Free PMC article. Review.
-
Identification of Potato StPIN Gene Family and Regulation of Root Development by StPIN4.Int J Mol Sci. 2024 Oct 26;25(21):11517. doi: 10.3390/ijms252111517. Int J Mol Sci. 2024. PMID: 39519072 Free PMC article.
-
Modeling of the Potential Geographical Distribution of Three Fritillaria Species Under Climate Change.Front Plant Sci. 2022 Jan 10;12:749838. doi: 10.3389/fpls.2021.749838. eCollection 2021. Front Plant Sci. 2022. PMID: 35082804 Free PMC article.
-
Insights on Phytohormonal Crosstalk in Plant Response to Nitrogen Stress: A Focus on Plant Root Growth and Development.Int J Mol Sci. 2023 Feb 11;24(4):3631. doi: 10.3390/ijms24043631. Int J Mol Sci. 2023. PMID: 36835044 Free PMC article. Review.
-
Integration of reactive oxygen species and nutrient signalling to shape root system architecture.Plant Cell Environ. 2023 Feb;46(2):379-390. doi: 10.1111/pce.14504. Epub 2022 Dec 13. Plant Cell Environ. 2023. PMID: 36479711 Free PMC article. Review.
References
-
- Sengupta D., Reddy A.R. Simplifying the root dynamics: From complex hormone—Environment interactions to specific root architectural modulation. Plant Growth Regul. 2018;85:337–349. doi: 10.1007/s10725-018-0397-1. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

