Significance of Heme and Heme Degradation in the Pathogenesis of Acute Lung and Inflammatory Disorders
- PMID: 34073678
- PMCID: PMC8197128
- DOI: 10.3390/ijms22115509
Significance of Heme and Heme Degradation in the Pathogenesis of Acute Lung and Inflammatory Disorders
Abstract
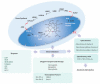
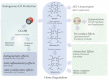
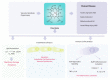
The heme molecule serves as an essential prosthetic group for oxygen transport and storage proteins, as well for cellular metabolic enzyme activities, including those involved in mitochondrial respiration, xenobiotic metabolism, and antioxidant responses. Dysfunction in both heme synthesis and degradation pathways can promote human disease. Heme is a pro-oxidant via iron catalysis that can induce cytotoxicity and injury to the vascular endothelium. Additionally, heme can modulate inflammatory and immune system functions. Thus, the synthesis, utilization and turnover of heme are by necessity tightly regulated. The microsomal heme oxygenase (HO) system degrades heme to carbon monoxide (CO), iron, and biliverdin-IXα, that latter which is converted to bilirubin-IXα by biliverdin reductase. Heme degradation by heme oxygenase-1 (HO-1) is linked to cytoprotection via heme removal, as well as by activity-dependent end-product generation (i.e., bile pigments and CO), and other potential mechanisms. Therapeutic strategies targeting the heme/HO-1 pathway, including therapeutic modulation of heme levels, elevation (or inhibition) of HO-1 protein and activity, and application of CO donor compounds or gas show potential in inflammatory conditions including sepsis and pulmonary diseases.
Keywords: acute lung injury; carbon monoxide; heme; heme oxygenase; inflammation; lung disease; sepsis.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders.Antioxidants (Basel). 2022 Mar 15;11(3):555. doi: 10.3390/antiox11030555. Antioxidants (Basel). 2022. PMID: 35326205 Free PMC article. Review.
-
Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation.Transl Res. 2016 Jan;167(1):7-34. doi: 10.1016/j.trsl.2015.06.011. Epub 2015 Jun 23. Transl Res. 2016. PMID: 26166253 Free PMC article. Review.
-
Heme oxygenase-1/carbon monoxide as modulators of autophagy and inflammation.Arch Biochem Biophys. 2019 Dec 15;678:108186. doi: 10.1016/j.abb.2019.108186. Epub 2019 Nov 5. Arch Biochem Biophys. 2019. PMID: 31704095 Review.
-
Heme oxygenase-1/carbon monoxide: from metabolism to molecular therapy.Am J Respir Cell Mol Biol. 2009 Sep;41(3):251-60. doi: 10.1165/rcmb.2009-0170TR. Epub 2009 Jul 17. Am J Respir Cell Mol Biol. 2009. PMID: 19617398 Free PMC article. Review.
-
Heme oxygenase-1 expression in disease states.Acta Biochim Pol. 2005;52(2):273-84. Epub 2005 May 31. Acta Biochim Pol. 2005. PMID: 15933765 Review.
Cited by
-
Carbon monoxide refines ovarian structure changes and attenuates oxidative stress via modulating of heme oxygenase system in a rat model of polycystic ovary syndrome: An experimental study.Int J Reprod Biomed. 2024 Oct 14;22(8):627-638. doi: 10.18502/ijrm.v22i8.17231. eCollection 2024 Aug. Int J Reprod Biomed. 2024. PMID: 39494120 Free PMC article.
-
Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders.Antioxidants (Basel). 2022 Mar 15;11(3):555. doi: 10.3390/antiox11030555. Antioxidants (Basel). 2022. PMID: 35326205 Free PMC article. Review.
-
Regulatory mechanisms of heme regulatory protein BACH1: a potential therapeutic target for cancer.Med Oncol. 2021 Sep 4;38(10):122. doi: 10.1007/s12032-021-01573-z. Med Oncol. 2021. PMID: 34482423 Review.
-
New Insights Regarding Hemin Inhibition of the Purified Rat Brain 2-Oxoglutarate Carrier and Relationships with Mitochondrial Dysfunction.J Clin Med. 2022 Dec 19;11(24):7519. doi: 10.3390/jcm11247519. J Clin Med. 2022. PMID: 36556135 Free PMC article.
-
Effects of Dietary Ferroporphyrin Supplementation on Growth Performance, Antioxidant Capacity, Immune Response, and Oxygen-Carrying Capacity in Gibel Carp (Carassius auratus gibelio).Animals (Basel). 2024 Oct 28;14(21):3104. doi: 10.3390/ani14213104. Animals (Basel). 2024. PMID: 39518827 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

