Sex-Specific Cannabidiol- and Iloperidone-Induced Neuronal Activity Changes in an In Vitro MAM Model System of Schizophrenia
- PMID: 34073710
- PMCID: PMC8197248
- DOI: 10.3390/ijms22115511
Sex-Specific Cannabidiol- and Iloperidone-Induced Neuronal Activity Changes in an In Vitro MAM Model System of Schizophrenia
Abstract
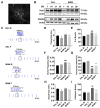
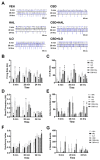
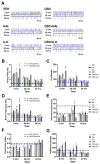
Cortical circuit dysfunction is thought to be an underlying mechanism of schizophrenia (SZ) pathophysiology with normalization of aberrant circuit activity proposed as a biomarker for antipsychotic efficacy. Cannabidiol (CBD) shows potential as an adjunctive antipsychotic therapy; however, potential sex effects in these drug interactions remain unknown. In the present study, we sought to elucidate sex effects of CBD coadministration with the atypical antipsychotic iloperidone (ILO) on the activity of primary cortical neuron cultures derived from the rat methylazoxymethanol acetate (MAM) model used for the study of SZ. Spontaneous network activity measurements were obtained using a multielectrode array at baseline and following administration of CBD or ILO alone, or combined. At baseline, MAM male neurons displayed increased bursting activity whereas MAM female neurons exhibited no difference in bursting activity compared to sex-matched controls. CBD administered alone showed a rapid but transient increase in neuronal activity in the MAM networks, an effect more pronounced in females. Furthermore, ILO had an additive effect on CBD-induced elevations in activity in the MAM male neurons. In the MAM female neurons, CBD or ILO administration resulted in time-dependent elevations in neuronal activity, but the short-term CBD-induced increases in activity were lost when CBD and ILO were combined. Our findings indicate that CBD induces rapid increases in cortical neuronal activity, with sex-specific drug interactions upon ILO coadministration. This suggests that sex should be a consideration when implementing adjunct therapy for treatment of SZ.
Keywords: cannabidiol; electrophysiology; haloperidol; iloperidone; primary cortical neurons; schizophrenia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; Washington, DC, USA: 2013. Schizophrenia and other psychotic disorders.
-
- Nielsen R.E., Levander S., Kjaersdam Telleus G., Jensen S.O., Ostergaard Christensen T., Leucht S. Second-generation antipsychotic effect on cognition in patients with schizophrenia—A meta-analysis of randomized clinical trials. Acta Psychiatr. Scand. 2015;131:185–196. doi: 10.1111/acps.12374. - DOI - PubMed
-
- Howard C.H., Fiedosewicz H., Patel C., Klegon D.A., Bayog R., Berman I. Treatment response and gender in patients with schizophrenia and schizoaffective disorder. Schizophr. Res. 2001;49:232.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

