Co-Adaptation of Physical Attributes of the Mammalian Female Reproductive Tract and Sperm to Facilitate Fertilization
- PMID: 34073739
- PMCID: PMC8225031
- DOI: 10.3390/cells10061297
Co-Adaptation of Physical Attributes of the Mammalian Female Reproductive Tract and Sperm to Facilitate Fertilization
Abstract
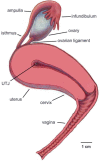
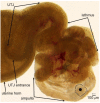
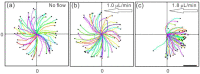
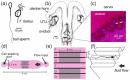
The functions of the female reproductive tract not only encompass sperm migration, storage, and fertilization, but also support the transport and development of the fertilized egg through to the birth of offspring. Further, because the tract is open to the external environment, it must also provide protection against invasive pathogens. In biophysics, sperm are considered "pusher microswimmers", because they are propelled by pushing fluid behind them. This type of swimming by motile microorganisms promotes the tendency to swim along walls and upstream in gentle fluid flows. Thus, the architecture of the walls of the female tract, and the gentle flows created by cilia, can guide sperm migration. The viscoelasticity of the fluids in the tract, such as mucus secretions, also promotes the cooperative swimming of sperm that can improve fertilization success; at the same time, the mucus can also impede the invasion of pathogens. This review is focused on how the mammalian female reproductive tract and sperm interact physically to facilitate the movement of sperm to the site of fertilization. Knowledge of female/sperm interactions can not only explain how the female tract can physically guide sperm to the fertilization site, but can also be applied for the improvement of in vitro fertilization devices.
Keywords: cervix; fertilization; oviduct; sperm; uterus; vagina.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

