Single Ventricle-A Comprehensive Review
- PMID: 34073809
- PMCID: PMC8225092
- DOI: 10.3390/children8060441
Single Ventricle-A Comprehensive Review
Abstract
In this paper, the author enumerates cardiac defects with a functionally single ventricle, summarizes single ventricle physiology, presents a summary of management strategies to address the single ventricle defects, goes over the steps of staged total cavo-pulmonary connection, cites the prevalence of inter-stage mortality, names the causes of inter-stage mortality, discusses strategies to address the inter-stage mortality, reviews post-Fontan issues, and introduces alternative approaches to Fontan circulation.
Keywords: Down syndrome; Fontan circulation; biventricular repair; double-inlet left ventricle; heterotaxy syndromes; hypoplastic left heart syndrome; mitral atresia with normal aortic root; single ventricle; tricuspid atresia; unbalanced atrioventricular septal defect.
Conflict of interest statement
The author declares no conflict of interest.
Figures
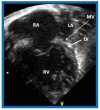

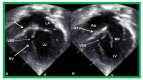







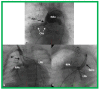





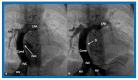










References
-
- Rao P.S., Striepe V., Kambam J. Hypoplastic left heart syndrome. In: Kambam J., editor. Cardiac Anesthesia for Infants and Children. Mosby-Year Book; St. Louis, MO, USA: 1994. pp. 296–309.
-
- Alapati S., Rao P.S. Hypoplastic Left Heart Syndrome in the Neonate. Neonatol. Today. 2011;6:1–9.
-
- Rao P.S., Alapati S. Hypoplastic left heart syndrome. In: Vijayalakshmi I.B., Rao P.S., Chugh R., editors. A Comprehensive Approach to Management of Congenital Heart Diseases. Jaypee Publications; New Delhi, India: 2013. pp. 662–678.
Publication types
LinkOut - more resources
Full Text Sources

