Environmental Factors-Induced Oxidative Stress: Hormonal and Molecular Pathway Disruptions in Hypogonadism and Erectile Dysfunction
- PMID: 34073826
- PMCID: PMC8225220
- DOI: 10.3390/antiox10060837
Environmental Factors-Induced Oxidative Stress: Hormonal and Molecular Pathway Disruptions in Hypogonadism and Erectile Dysfunction
Abstract
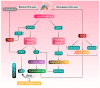
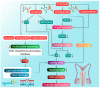
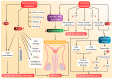
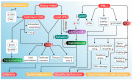
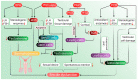
Hypogonadism is an endocrine disorder characterized by inadequate serum testosterone production by the Leydig cells of the testis. It is triggered by alterations in the hypothalamic-pituitary-gonadal axis. Erectile dysfunction (ED) is another common disorder in men that involves an alteration in erectile response-organic, relational, or psychological. The incidence of hypogonadism and ED is common in men aged over 40 years. Hypogonadism (including late-onset hypogonadism) and ED may be linked to several environmental factors-induced oxidative stresses. The factors mainly include exposure to pesticides, radiation, air pollution, heavy metals and other endocrine-disrupting chemicals. These environmental risk factors may induce oxidative stress and lead to hormonal dysfunctions. To better understand the subject, the study used many keywords, including "hypogonadism", "late-onset hypogonadism", "testosterone", "erectile dysfunction", "reactive oxygen species", "oxidative stress", and "environmental pollution" in major online databases, such as SCOPUS and PUBMED to extract relevant scientific information. Based on these parameters, this review summarizes a comprehensive insight into the important environmental issues that may have a direct or indirect association with hypogonadism and ED in men. The study concludes that environmental factors-induced oxidative stress may cause infertility in men. The hypothesis and outcomes were reviewed critically, and the mechanistic approaches are applied through oxidant-sensitive pathways. This study also provides reccomendations on future therapeutic interventions and protective measures against such adverse environmental factors-induced hypogonadism and ED.
Keywords: air pollution; endocrine-disrupting chemicals; erectile dysfunction; heavy metals; hypogonadism; infertility; pesticide; radiation; testosterone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Pituitary radiographic abnormalities and clinical correlates of hypogonadism in elderly males presenting with erectile dysfunction.Aging Male. 2002 Mar;5(1):38-46. Aging Male. 2002. PMID: 12040974
-
Erectile dysfunction is a prognostic indicator of comorbidities in men with late onset hypogonadism.Aging Male. 2015;18(3):186-94. doi: 10.3109/13685538.2015.1046044. Epub 2015 Jun 1. Aging Male. 2015. PMID: 26030350
-
Urological Survivorship Issues Among Adolescent Boys and Young Men Who Are Cancer Survivors.Sex Med Rev. 2018 Jul;6(3):396-409. doi: 10.1016/j.sxmr.2017.12.007. Epub 2018 Feb 1. Sex Med Rev. 2018. PMID: 29396283 Review.
-
Long-term treatment with testosterone undecanoate injections in men with hypogonadism alleviates erectile dysfunction and reduces risk of major adverse cardiovascular events, prostate cancer, and mortality.Aging Male. 2020 Mar;23(1):81-92. doi: 10.1080/13685538.2019.1575354. Epub 2019 Feb 20. Aging Male. 2020. PMID: 30782054
-
Testosterone therapy in erectile dysfunction and hypogonadism.J Sex Med. 2005 Nov;2(6):785-92. doi: 10.1111/j.1743-6109.2005.00139.x. J Sex Med. 2005. PMID: 16422803 Review.
Cited by
-
Harnessing the power of nutritional antioxidants against adrenal hormone imbalance-associated oxidative stress.Front Endocrinol (Lausanne). 2023 Nov 30;14:1271521. doi: 10.3389/fendo.2023.1271521. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38098868 Free PMC article. Review.
-
Recent Publication Trends in Radiotherapy and Male Infertility over Two Decades: A Scientometric Analysis.Front Cell Dev Biol. 2022 May 12;10:877079. doi: 10.3389/fcell.2022.877079. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35646894 Free PMC article.
-
The Mechanisms and Management of Age-Related Oxidative Stress in Male Hypogonadism Associated with Non-communicable Chronic Disease.Antioxidants (Basel). 2021 Nov 18;10(11):1834. doi: 10.3390/antiox10111834. Antioxidants (Basel). 2021. PMID: 34829704 Free PMC article. Review.
-
Exploring the Molecular Link Between Diabetes and Erectile Dysfunction Through Single-Cell Transcriptome Analysis.Genes (Basel). 2024 Dec 13;15(12):1596. doi: 10.3390/genes15121596. Genes (Basel). 2024. PMID: 39766863 Free PMC article. Review.
-
Association between the oxidative balance score and testosterone deficiency: a cross-sectional study of the NHANES, 2011-2016.Sci Rep. 2025 Mar 7;15(1):8040. doi: 10.1038/s41598-025-92934-5. Sci Rep. 2025. PMID: 40055483 Free PMC article.
References
-
- Tüttelmann F., Nieschlag E. Classification of andrological disorders. In: Nieschlag E., Behre H.M., Nieschlag S., editors. Andrology, Male Reproductive Health and Dysfucntion. 3rd ed. Springer; Berlin/Heidelberg, Germany: 2010. pp. 87–92.
-
- Sizar O., Schwartz J. Hypogonadism. Stat Pearls Publishing; Treasure Island, FL, USA: 2019.
-
- Kim K.M. Late-onset hypogonadism. Korean J. Fam. Pract. 2013;3:245–254.
Publication types
LinkOut - more resources
Full Text Sources
Medical

