P2Y2R Deficiency Ameliorates Hepatic Steatosis by Reducing Lipogenesis and Enhancing Fatty Acid β-Oxidation through AMPK and PGC-1α Induction in High-Fat Diet-Fed Mice
- PMID: 34073834
- PMCID: PMC8197197
- DOI: 10.3390/ijms22115528
P2Y2R Deficiency Ameliorates Hepatic Steatosis by Reducing Lipogenesis and Enhancing Fatty Acid β-Oxidation through AMPK and PGC-1α Induction in High-Fat Diet-Fed Mice
Abstract
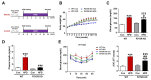
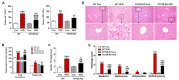
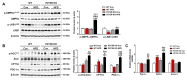
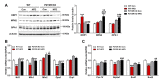
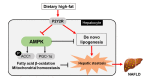
Non-alcoholic fatty liver disease (NAFLD) is a chronic metabolic liver disease associated with obesity and insulin resistance. Activation of the purinergic receptor P2Y2R has been reported to promote adipogenesis, inflammation and dyslipidemia in adipose tissues in obese mice. However, the role of P2Y2R and its mechanisms in NAFLD remain unknown. We hypothesized that P2Y2R deficiency may play a protective role in NAFLD by modulating lipid metabolism in the liver. In this study, we fed wild type and P2Y2R knockout mice with a high-fat diet (HFD) for 12 weeks and analyzed metabolic phenotypes. First, P2Y2R deficiency effectively improved insulin resistance with a reduction in body weight and plasma insulin. Second, P2Y2R deficiency attenuated hepatic lipid accumulation and injury with reduced alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels. Third, P2Y2R deficiency decreased the expression of fatty acid synthesis mediators (cluster of differentiation (CD36), fatty acid synthase (FAS), and stearoyl-CoA desaturase 1 (SCD1)); and increased the expression of adipose triglyceride lipase (ATGL), a lipolytic enzyme. Mechanistically, P2Y2R deficiency increased the AMP-activated protein kinase (AMPK) activity to improve mitochondrial fatty acid β-oxidation (FAO) by regulating acetyl-CoA carboxylase (ACC) and carnitine palmitoyltransferase 1A (CPT1A)-mediated FAO pathway. In addition, P2Y2R deficiency increased peroxisome proliferator-activated gamma co-activator-1α (PGC-1α)-mediated mitochondrial biogenesis. Conclusively, P2Y2R deficiency ameliorated HFD-induced hepatic steatosis by enhancing FAO through AMPK signaling and PGC-1α pathway, suggesting P2Y2R as a promising therapeutic target for NAFLD.
Keywords: AMPK; NAFLD; P2Y2R; fatty acid β-oxidation; hepatic steatosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
LB100 ameliorates nonalcoholic fatty liver disease via the AMPK/Sirt1 pathway.World J Gastroenterol. 2019 Dec 7;25(45):6607-6618. doi: 10.3748/wjg.v25.i45.6607. World J Gastroenterol. 2019. PMID: 31832001 Free PMC article.
-
Crocin ameliorates hepatic steatosis through activation of AMPK signaling in db/db mice.Lipids Health Dis. 2019 Jan 8;18(1):11. doi: 10.1186/s12944-018-0955-6. Lipids Health Dis. 2019. PMID: 30621686 Free PMC article.
-
Chronic administration of recombinant IL-6 upregulates lipogenic enzyme expression and aggravates high-fat-diet-induced steatosis in IL-6-deficient mice.Dis Model Mech. 2015 Jul 1;8(7):721-31. doi: 10.1242/dmm.019166. Epub 2015 May 14. Dis Model Mech. 2015. PMID: 26035386 Free PMC article.
-
Free radical biology for medicine: learning from nonalcoholic fatty liver disease.Free Radic Biol Med. 2013 Dec;65:952-968. doi: 10.1016/j.freeradbiomed.2013.08.174. Epub 2013 Aug 29. Free Radic Biol Med. 2013. PMID: 23994574 Review.
-
n-3 Polyunsaturated fatty acids for the management of alcoholic liver disease: A critical review.Crit Rev Food Sci Nutr. 2019;59(sup1):S116-S129. doi: 10.1080/10408398.2018.1544542. Epub 2018 Dec 22. Crit Rev Food Sci Nutr. 2019. PMID: 30580553 Review.
Cited by
-
PGC-1α affects skeletal muscle and adipose tissue development by regulating mitochondrial biogenesis.Mol Genet Genomics. 2022 May;297(3):621-633. doi: 10.1007/s00438-022-01878-2. Epub 2022 Mar 15. Mol Genet Genomics. 2022. PMID: 35290519 Review.
-
Knockout of Purinergic P2Y6 Receptor Fails to Improve Liver Injury and Inflammation in Non-Alcoholic Steatohepatitis.Int J Mol Sci. 2023 Feb 14;24(4):3800. doi: 10.3390/ijms24043800. Int J Mol Sci. 2023. PMID: 36835211 Free PMC article.
-
Role of purinergic signalling in obesity-associated end-organ damage: focus on the effects of natural plant extracts.Front Endocrinol (Lausanne). 2023 Jul 5;14:1181948. doi: 10.3389/fendo.2023.1181948. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37476493 Free PMC article. Review.
-
Purinergic signaling in liver disease: calcium signaling and induction of inflammation.Purinergic Signal. 2025 Feb;21(1):69-81. doi: 10.1007/s11302-024-10044-9. Epub 2024 Sep 25. Purinergic Signal. 2025. PMID: 39320433 Free PMC article. Review.
-
Purinergic Signaling in Non-Parenchymal Liver Cells.Int J Mol Sci. 2024 Aug 30;25(17):9447. doi: 10.3390/ijms25179447. Int J Mol Sci. 2024. PMID: 39273394 Free PMC article. Review.
References
-
- Edmunds L.R., Xie B., Mills A.M., Huckestein B.R., Undamatla R., Murali A., Pangburn M.M., Martin J., Sipula I., Kaufman B.A., et al. Liver-specific Prkn knockout mice are more susceptible to diet-induced hepatic steatosis and insulin resistance. Mol. Metab. 2020;41:101051. doi: 10.1016/j.molmet.2020.101051. - DOI - PMC - PubMed
-
- Tong J., Han C.J., Zhang J.Z., He W.Z., Zhao G.J., Cheng X., Zhang L., Deng K.Q., Liu Y., Fan H.F., et al. Hepatic Interferon Regulatory Factor 6 Alleviates Liver Steatosis and Metabolic Disorder by Transcriptionally Suppressing Peroxisome Proliferator-Activated Receptor gamma in Mice. Hepatology. 2019;69:2471–2488. doi: 10.1002/hep.30559. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

