A Novel Method for Creating a Synthetic L-DOPA Proteome and In Vitro Evidence of Incorporation
- PMID: 34073856
- PMCID: PMC8162537
- DOI: 10.3390/proteomes9020024
A Novel Method for Creating a Synthetic L-DOPA Proteome and In Vitro Evidence of Incorporation
Abstract
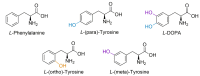
Proteinopathies are protein misfolding diseases that have an underlying factor that affects the conformation of proteoforms. A factor hypothesised to play a role in these diseases is the incorporation of non-protein amino acids into proteins, with a key example being the therapeutic drug levodopa. The presence of levodopa as a protein constituent has been explored in several studies, but it has not been examined in a global proteomic manner. This paper provides a proof-of-concept method for enzymatically creating levodopa-containing proteins using the enzyme tyrosinase and provides spectral evidence of in vitro incorporation in addition to the induction of the unfolded protein response due to levodopa.
Keywords: L-DOPA; Levodopa; PTM; misincorporation; post-translational modifications.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
L-DOPA causes mitochondrial dysfunction in vitro: A novel mechanism of L-DOPA toxicity uncovered.Int J Biochem Cell Biol. 2019 Dec;117:105624. doi: 10.1016/j.biocel.2019.105624. Epub 2019 Oct 22. Int J Biochem Cell Biol. 2019. PMID: 31654750
-
Evidence for L-dopa incorporation into cell proteins in patients treated with levodopa.J Neurochem. 2006 Aug;98(4):1061-7. doi: 10.1111/j.1471-4159.2006.03941.x. Epub 2006 Jun 12. J Neurochem. 2006. PMID: 16771833
-
Current Advances in L-DOPA and DOPA-Peptidomimetics: Chemistry, Applications and Biological Activity.Curr Med Chem. 2015;22(36):4138-65. doi: 10.2174/0929867322666150625095748. Curr Med Chem. 2015. PMID: 26112144 Review.
-
Dopamine- or L-DOPA-induced neurotoxicity: the role of dopamine quinone formation and tyrosinase in a model of Parkinson's disease.Neurotox Res. 2003;5(3):165-76. doi: 10.1007/BF03033137. Neurotox Res. 2003. PMID: 12835121 Review.
-
Post-translational incorporation of 3,4-dihydroxyphenylalanine into the C terminus of α-tubulin in living cells.FEBS J. 2018 Mar;285(6):1064-1078. doi: 10.1111/febs.14386. Epub 2018 Feb 13. FEBS J. 2018. PMID: 29341414
Cited by
-
L-DOPA Autoxidation: An Empirical Valence Bond Simulation of the Reactive Step.J Phys Chem B. 2024 Sep 5;128(35):8355-8361. doi: 10.1021/acs.jpcb.4c03002. Epub 2024 Aug 24. J Phys Chem B. 2024. PMID: 39180475 Free PMC article.
-
Enzymatic Phosphorylation of Oxidized Tyrosine Residues.J Proteome Res. 2023 Jun 2;22(6):1959-1968. doi: 10.1021/acs.jproteome.3c00061. Epub 2023 May 5. J Proteome Res. 2023. PMID: 37146082 Free PMC article.
References
-
- Alexander T., Sortwell C.E., Sladek C.D., Roth R.H., Steece-Collier K. Comparison of neurotoxicity following repeated administration of L-dopa, D-dopa, and dopamine to embryonic mesencephalic dopamine neurons in cultures derived from Fisher 344 and Sprague-Dawley donors. Cell Transplant. 1997;6:309–315. doi: 10.1177/096368979700600313. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

