Management of Acute Lung Injury: Palmitoylethanolamide as a New Approach
- PMID: 34073872
- PMCID: PMC8197255
- DOI: 10.3390/ijms22115533
Management of Acute Lung Injury: Palmitoylethanolamide as a New Approach
Abstract
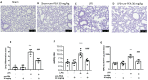
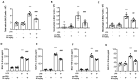
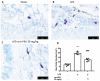
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are common and devastating clinical disorders with high mortality and no specific therapy. Lipopolysaccharide (LPS) is usually used intratracheally to induce ALI in mice. The aim of this study was to examine the effects of an ultramicronized preparation of palmitoylethanolamide (um-PEA) in mice subjected to LPS-induced ALI. Histopathological analysis reveals that um-PEA reduced alteration in lung after LPS intratracheal administration. Besides, um-PEA decreased wet/dry weight ratio and myeloperoxidase, a marker of neutrophils infiltration, macrophages and total immune cells number and mast cells degranulation in lung. Moreover, um-PEA could also decrease cytokines release of interleukin (IL)-6, interleukin (IL)-1β, tumor necrosis factor (TNF)-α and interleukin (IL)-18. Furthermore, um-PEA significantly inhibited the phosphorylation of nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha (IκBα) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation in ALI, and at the same time decreased extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (p38/MAPK) expression, that was increased after LPS administration. Our study suggested that um-PEA contrasted LPS-induced ALI, exerting its potential role as an adjuvant anti-inflammatory therapeutic for treating lung injury, maybe also by p38/NF-κB pathway.
Keywords: acute lung injury; cytokines; inflammation; palmitoylethanolamide; ultramicronized.
Conflict of interest statement
Salvatore Cuzzocrea is a coinventor on patent WO2013121449 A8 (Epitech Group s.r.l.), which deals with methods and compositions for the modulation of amidases capable of hydrolyzing N-acylethanolamines employable in the treatment of inflammatory diseases. This invention is wholly unrelated to the present study. Moreover, Cuzzocrea is also, with Epitech Group, a coinventor on the patents EP 2 821 083, MI2014 A001495, and 102015000067344, which are unrelated to the study. The remaining authors report no conflicts of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

