Chemical Synthesis and Structure-Activity Relationship Study Yield Desotamide a Analogues with Improved Antibacterial Activity
- PMID: 34073984
- PMCID: PMC8225045
- DOI: 10.3390/md19060303
Chemical Synthesis and Structure-Activity Relationship Study Yield Desotamide a Analogues with Improved Antibacterial Activity
Abstract
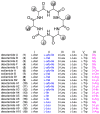
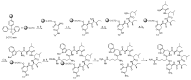
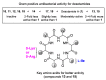
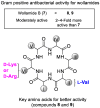
Desotamides A, a cyclohexapeptide produced by the deep-sea-derived Streptomyces scopuliridis SCSIO ZJ46, displays notable antibacterial activities against strains of Streptococcus pnuemoniae, Staphylococcus aureus, and methicillin-resistant Staphylococcus epidermidis (MRSE). In this study, to further explore its antibacterial potential and reveal the antibacterial structure-activity relationship of desotamides, 13 cyclopeptides including 10 new synthetic desotamide A analogues and wollamides B/B1/B2 were synthesized and evaluated for their antibacterial activities against a panel of Gram-positive and -negative pathogens. The bioactivity data reveal that residues at position II and VI greatly impact antibacterial activity. The most potent antibacterial analogues are desotamide A4 (13) and A6 (15) where l-allo-Ile at position II was substituted with l-Ile and Gly at position VI was simultaneously replaced by d-Lys or d-Arg; desotamides A4 (13) and A6 (15) showed a 2-4-fold increase of antibacterial activities against a series of Gram-positive pathogens including the prevalent clinical drug-resistant pathogen methicillin-resistant Staphylococcus aureus (MRSA) with MIC values of 8-32 μg/mL compared to the original desotamide A. The enhanced antibacterial activity, broad antibacterial spectrum of desotamides A4 and A6 highlighted their potential as new antibiotic leads for further development.
Keywords: antibacterial; cyclohexapeptides; desotamides; drug-resistant bacteria; solid-phase peptide synthesis; structure-activity relationship.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Cyclic Hexapeptides from the Deep South China Sea-Derived Streptomyces scopuliridis SCSIO ZJ46 Active Against Pathogenic Gram-Positive Bacteria.J Nat Prod. 2014 Aug 22;77(8):1937-41. doi: 10.1021/np500399v. J Nat Prod. 2014. PMID: 25072108
-
Total Synthesis and Antibacterial Study of Cyclohexapeptides Desotamide B, Wollamide B and Their Analogs.Chem Biodivers. 2018 Jan;15(1). doi: 10.1002/cbdv.201700414. Epub 2017 Dec 27. Chem Biodivers. 2018. PMID: 29125222
-
The roles of genes associated with regulation, transportation, and macrocyclization in desotamide biosynthesis in Streptomyces scopuliridis SCSIO ZJ46.Appl Microbiol Biotechnol. 2020 Mar;104(6):2603-2610. doi: 10.1007/s00253-020-10414-4. Epub 2020 Jan 31. Appl Microbiol Biotechnol. 2020. PMID: 32002605
-
In-vitro profile of a new beta-lactam, ceftobiprole, with activity against methicillin-resistant Staphylococcus aureus.Clin Microbiol Infect. 2007 Jun;13 Suppl 2:17-24. doi: 10.1111/j.1469-0691.2007.01722.x. Clin Microbiol Infect. 2007. PMID: 17488372 Review.
-
Discovery, synthesis, and optimization of teixobactin, a novel antibiotic without detectable bacterial resistance.J Pept Sci. 2022 Nov;28(11):e3428. doi: 10.1002/psc.3428. Epub 2022 Jun 13. J Pept Sci. 2022. PMID: 35610021 Review.
Cited by
-
Recent Advances in Polypeptide Antibiotics Derived from Marine Microorganisms.Mar Drugs. 2023 Oct 22;21(10):547. doi: 10.3390/md21100547. Mar Drugs. 2023. PMID: 37888482 Free PMC article. Review.
-
Structure-activity relationships andz interindividual variability of drug responses: pharmacogenomics with antimicrobial drugs as a paradigm.J Int Med Res. 2023 Nov;51(11):3000605231214065. doi: 10.1177/03000605231214065. J Int Med Res. 2023. PMID: 38019107 Free PMC article. Review.
-
Optimizing the Synthesis of Desotamides and Related Cyclic Antimicrobial Peptides.Methods Mol Biol. 2025;2931:299-311. doi: 10.1007/978-1-0716-4562-8_23. Methods Mol Biol. 2025. PMID: 40531466
References
-
- Medina E., Pieper D.H. Tackling Threats and Future Problems of Multidrug-Resistant Bacteria. Curr. Top Microbiol. Immunol. 2016;398:3–33. - PubMed
-
- O’Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Review on Antimicrobial Resistance. Wellcome Trust and HM Government; London, UK: 2016. [(accessed on 1 May 2021)]. Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20c....
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

