ERO1-PDI Redox Signaling in Health and Disease
- PMID: 34074138
- PMCID: PMC8817699
- DOI: 10.1089/ars.2021.0018
ERO1-PDI Redox Signaling in Health and Disease
Abstract
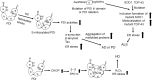
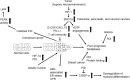
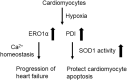
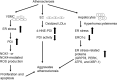
Significance: Protein disulfide isomerase (PDI) and endoplasmic reticulum oxidoreductase 1 (ERO1) are crucial for oxidative protein folding in the endoplasmic reticulum (ER). These enzymes are frequently overexpressed and secreted, and they contribute to the pathology of neurodegenerative, cardiovascular, and metabolic diseases. Recent Advances: Tissue-specific knockout mouse models and pharmacologic inhibitors have been developed to advance our understanding of the cell-specific functions of PDI and ERO1. In addition to their roles in protecting cells from the unfolded protein response and oxidative stress, recent studies have revealed that PDI and ERO1 also function outside of the cells. Critical Issues: Despite the well-known contributions of PDI and ERO1 to specific disease pathology, the detailed molecular and cellular mechanisms underlying these activities remain to be elucidated. Further, although PDI and ERO1 inhibitors have been identified, the results from previous studies require careful evaluation, as many of these agents are not selective and may have significant cytotoxicity. Future Directions: The functions of PDI and ERO1 in the ER have been extensively studied. Additional studies will be required to define their functions outside the ER.
Keywords: ERO1; PDI; disease; inhibitors; oxidative ER stress; unfolded protein response.
Conflict of interest statement
Z.A. and K.L.O. are employees of Cayman Chemical Company Inc. The other authors declare no competing financial interests.
Figures



Similar articles
-
Role of the ERO1-PDI interaction in oxidative protein folding and disease.Pharmacol Ther. 2020 Jun;210:107525. doi: 10.1016/j.pharmthera.2020.107525. Epub 2020 Mar 20. Pharmacol Ther. 2020. PMID: 32201313 Free PMC article. Review.
-
Ero1-PDI interactions, the response to redox flux and the implications for disulfide bond formation in the mammalian endoplasmic reticulum.Philos Trans R Soc Lond B Biol Sci. 2013 Mar 25;368(1617):20110403. doi: 10.1098/rstb.2011.0403. Print 2013 May 5. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 23530257 Free PMC article.
-
ERO1: A protein disulfide oxidase and H2O2 producer.Free Radic Biol Med. 2015 Jun;83:299-304. doi: 10.1016/j.freeradbiomed.2015.01.011. Epub 2015 Jan 31. Free Radic Biol Med. 2015. PMID: 25651816 Review.
-
Molecular mechanisms regulating oxidative activity of the Ero1 family in the endoplasmic reticulum.Antioxid Redox Signal. 2010 Oct;13(8):1177-87. doi: 10.1089/ars.2010.3230. Antioxid Redox Signal. 2010. PMID: 20486761 Review.
-
Ero1-α and PDIs constitute a hierarchical electron transfer network of endoplasmic reticulum oxidoreductases.J Cell Biol. 2013 Sep 16;202(6):861-74. doi: 10.1083/jcb.201303027. J Cell Biol. 2013. PMID: 24043701 Free PMC article.
Cited by
-
Altered tear fluid protein expression in persons with mild Alzheimer's disease in proteins involved in oxidative stress, protein synthesis, and energy metabolism.J Alzheimers Dis. 2025 May;105(1):292-301. doi: 10.1177/13872877251326868. Epub 2025 Apr 4. J Alzheimers Dis. 2025. PMID: 40183343 Free PMC article.
-
Endoplasmic reticulum stress alters myelin associated protein expression and extracellular vesicle composition in human oligodendrocytes.Front Mol Biosci. 2024 Oct 1;11:1432945. doi: 10.3389/fmolb.2024.1432945. eCollection 2024. Front Mol Biosci. 2024. PMID: 39411401 Free PMC article.
-
Oxidative Stress: Signaling Pathways, Biological Functions, and Disease.MedComm (2020). 2025 Jul 1;6(7):e70268. doi: 10.1002/mco2.70268. eCollection 2025 Jul. MedComm (2020). 2025. PMID: 40599237 Free PMC article. Review.
-
Excessive or sustained endoplasmic reticulum stress: one of the culprits of adipocyte dysfunction in obesity.Ther Adv Endocrinol Metab. 2024 Oct 7;15:20420188241282707. doi: 10.1177/20420188241282707. eCollection 2024. Ther Adv Endocrinol Metab. 2024. PMID: 39381518 Free PMC article. Review.
-
Interplay of hypoxia, immune dysregulation, and metabolic stress in pathophysiology of type 1 diabetes.Front Immunol. 2025 Jun 4;16:1599321. doi: 10.3389/fimmu.2025.1599321. eCollection 2025. Front Immunol. 2025. PMID: 40534855 Free PMC article. Review.
References
-
- Abad Rico JI, Llau Pitarch JV, and Rocha E. Overview of venous thromboembolism. Drugs 70 Suppl 2: 3–10, 2010. - PubMed
-
- Abe JI, Ko KA, Kotla S, Wang Y, Paez-Mayorga J, Shin IJ, Imanishi M, Vu HT, Tao Y, Leiva-Juarez MM, Thomas TN, Medina JL, Won JH, Fujii Y, Giancursio CJ, McBeath E, Shin JH, Guzman L, Abe RJ, Taunton J, Mochizuki N, Faubion W, Cooke JP, Fujiwara K, Evans SE, and Le NT. MAGI1 as a link between endothelial activation and ER stress drives atherosclerosis. JCI Insight 4: e125570, 2019. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

