Longitudinal Neurocognitive Effects of Combined Electroconvulsive Therapy (ECT) and Pharmacotherapy in Major Depressive Disorder in Older Adults: Phase 2 of the PRIDE Study
- PMID: 34074611
- PMCID: PMC8595359
- DOI: 10.1016/j.jagp.2021.04.006
Longitudinal Neurocognitive Effects of Combined Electroconvulsive Therapy (ECT) and Pharmacotherapy in Major Depressive Disorder in Older Adults: Phase 2 of the PRIDE Study
Abstract
Objective: There is limited information regarding neurocognitive outcomes of right unilateral ultrabrief pulse width electroconvulsive therapy (RUL-UB ECT) combined with pharmacotherapy in older adults with major depressive disorder. We report longitudinal neurocognitive outcomes from Phase 2 of the Prolonging Remission in Depressed Elderly (PRIDE) study.
Method: After achieving remission with RUL-UB ECT and venlafaxine, older adults (≥60 years old) were randomized to receive symptom-titrated, algorithm-based longitudinal ECT (STABLE) plus pharmacotherapy (venlafaxine and lithium) or pharmacotherapy-only. A comprehensive neuropsychological battery was administered at baseline and throughout the 6-month treatment period. Statistical significance was defined as a p-value of less than 0.05 (two-sided test).
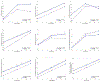
Results: With the exception of processing speed, there was statistically significant improvement across most neurocognitive measures from baseline to 6-month follow-up. There were no significant differences between the two treatment groups at 6 months on measures of psychomotor processing speed, autobiographical memory consistency, short-term and long-term verbal memory, phonemic fluency, inhibition, and complex visual scanning and cognitive flexibility.
Conclusion: To our knowledge, this is the first report of neurocognitive outcomes over a 6-month period of an acute course of RUL-UB ECT followed by one of 2 strategies to prolong remission in older adults with major depression. Neurocognitive outcome did not differ between STABLE plus pharmacotherapy versus pharmacotherapy alone over the 6-month continuation treatment phase. These findings support the safety of RUL-UB ECT in combination with pharmacotherapy in the prolonging of remission in late-life depression.
Trial registration: ClinicalTrials.gov NCT01028508.
Keywords: Electroconvulsive therapy; major depression; neurocognitive adverse effects.
Published by Elsevier Inc.
Conflict of interest statement
Disclosures/Conflicts of Interest (listed in alphabetical order)
Figures

Comment in
-
Improving ECT Outcomes in Late-Life Depression I.Am J Geriatr Psychiatry. 2022 Jan;30(1):29-31. doi: 10.1016/j.jagp.2021.05.013. Epub 2021 May 26. Am J Geriatr Psychiatry. 2022. PMID: 34162511 No abstract available.
References
-
- Spaans H-P, Sienaert P, Bouckaert F, et al. Speed of remission in elderly patients with depression: electroconvulsive therapy <em>v.</em> medication. The British Journal of Psychiatry 2015; 206:67–71 - PubMed
-
- Meyer JP, Swetter SK,Kellner CH: Electroconvulsive therapy in geriatric psychiatry: A selective review. Clinics in Geriatric Medicine In Press; - PubMed
-
- Socci C, Medda P, Toni C, et al. Electroconvulsive therapy and age: Age-related clinical features and effectiveness in treatment resistant major depressive episode. Journal of Affective Disorders 2018; 227:627–632 - PubMed
-
- Kumar S, Mulsant BH, Liu AY, et al. Systematic review of cognitive effects of electroconvulsive therapy in late-life depression. American Journal of Geriatric Psychiatry 2016; 24:547–565 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

