Respiratory syncytial virus induces β2-adrenergic receptor dysfunction in human airway smooth muscle cells
- PMID: 34074703
- PMCID: PMC8486349
- DOI: 10.1126/scisignal.abc1983
Respiratory syncytial virus induces β2-adrenergic receptor dysfunction in human airway smooth muscle cells
Abstract
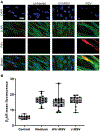
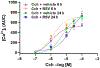
Pharmacologic agonism of the β2-adrenergic receptor (β2AR) induces bronchodilation by activating the enzyme adenylyl cyclase to generate cyclic adenosine monophosphate (cAMP). β2AR agonists are generally the most effective strategy to relieve acute airway obstruction in asthmatic patients, but they are much less effective when airway obstruction in young patients is triggered by infection with respiratory syncytial virus (RSV). Here, we investigated the effects of RSV infection on the abundance and function of β2AR in primary human airway smooth muscle cells (HASMCs) derived from pediatric lung tissue. We showed that RSV infection of HASMCs resulted in proteolytic cleavage of β2AR mediated by the proteasome. RSV infection also resulted in β2AR ligand-independent activation of adenylyl cyclase, leading to reduced cAMP synthesis compared to that in uninfected control cells. Last, RSV infection caused stronger airway smooth muscle cell contraction in vitro due to increased cytosolic Ca2+ concentrations. Thus, our results suggest that RSV infection simultaneously induces loss of functional β2ARs and activation of multiple pathways favoring airway obstruction in young patients, with the net effect of counteracting β2AR agonist-induced bronchodilation. These findings not only provide a potential mechanism for the reported lack of clinical efficacy of β2AR agonists for treating virus-induced wheezing but also open the path to developing more precise therapeutic strategies.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures





Similar articles
-
Defective Resensitization in Human Airway Smooth Muscle Cells Evokes β-Adrenergic Receptor Dysfunction in Severe Asthma.PLoS One. 2015 May 29;10(5):e0125803. doi: 10.1371/journal.pone.0125803. eCollection 2015. PLoS One. 2015. PMID: 26023787 Free PMC article.
-
Mechanisms of acute desensitization of the beta2AR-adenylyl cyclase pathway in human airway smooth muscle.Am J Respir Cell Mol Biol. 1998 Aug;19(2):338-48. doi: 10.1165/ajrcmb.19.2.3025. Am J Respir Cell Mol Biol. 1998. PMID: 9698608
-
Infant RSV infection desensitizes β2-adrenergic receptor via CXCL11-CXCR7 signaling in airway smooth muscle.bioRxiv [Preprint]. 2025 Jan 15:2025.01.13.632772. doi: 10.1101/2025.01.13.632772. bioRxiv. 2025. PMID: 39868223 Free PMC article. Preprint.
-
MicroRNAs: Mediators and Therapeutic Targets to Airway Hyper Reactivity After Respiratory Syncytial Virus Infection.Front Microbiol. 2018 Sep 11;9:2177. doi: 10.3389/fmicb.2018.02177. eCollection 2018. Front Microbiol. 2018. PMID: 30254626 Free PMC article. Review.
-
Contribution of neuroimmune mechanisms to airway inflammation and remodeling during and after respiratory syncytial virus infection.Pediatr Infect Dis J. 2003 Feb;22(2 Suppl):S66-74; discussion S74-5. doi: 10.1097/01.inf.0000053888.67311.1d. Pediatr Infect Dis J. 2003. PMID: 12671455 Review.
Cited by
-
A-Kinase-Anchoring Protein Subtypes Differentially Regulate GPCR Signaling and Function in Human Airway Smooth Muscle.Am J Respir Cell Mol Biol. 2025 Feb;72(2):133-144. doi: 10.1165/rcmb.2023-0358OC. Am J Respir Cell Mol Biol. 2025. PMID: 39141573
-
N-Acetyl-L-Cysteine Protects Airway Epithelial Cells during Respiratory Syncytial Virus Infection against Mucin Synthesis, Oxidative Stress, and Inflammatory Response and Inhibits HSPA6 Expression.Anal Cell Pathol (Amst). 2022 Aug 21;2022:4846336. doi: 10.1155/2022/4846336. eCollection 2022. Anal Cell Pathol (Amst). 2022. PMID: 36046596 Free PMC article.
-
The usual suspects: Respiratory syncytial virus and rhinovirus drive asthma development and exacerbation.Ann Allergy Asthma Immunol. 2025 Sep;135(3):261-267. doi: 10.1016/j.anai.2025.04.013. Epub 2025 Apr 25. Ann Allergy Asthma Immunol. 2025. PMID: 40287012 Review.
-
Update on the Role of β2AR and TRPV1 in Respiratory Diseases.Int J Mol Sci. 2024 Sep 24;25(19):10234. doi: 10.3390/ijms251910234. Int J Mol Sci. 2024. PMID: 39408565 Free PMC article. Review.
-
RSV-induced changes in a 3-dimensional organoid model of human fetal lungs.PLoS One. 2022 Mar 9;17(3):e0265094. doi: 10.1371/journal.pone.0265094. eCollection 2022. PLoS One. 2022. PMID: 35263387 Free PMC article.
References
-
- Kobilka BK, Dixon RA, Frielle T, Dohlman HG, Bolanowski MA, Sigal IS, Yang-Feng TL, Francke U, Caron MG, Lefkowitz RJ, cDNA for the human β2-adrenergic receptor: A protein with multiple membrane-spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor for platelet-derived growth factor. Proc. Natl. Acad. Sci. U.S.A 84, 46–50 (1987). - PMC - PubMed
-
- Barnes PJ, Basbaum CB, Nadel JA, Roberts JM, Localization of β-adrenoreceptors in mammalian lung by light microscopic autoradiography. Nature 299, 444–447 (1982). - PubMed
-
- Hamid QA, Mak JCW, Sheppard MN, Corrin B, Craig Venter J, Barnes PJ, Localization of β2-adrenoceptor messenger RNA in human and rat lung using in situ hybridization: Correlation with receptor autoradiography. Eur. J. Pharmacol 206, 133–138 (1991). - PubMed
-
- Shore SA, Moore PE, Regulation of β-adrenergic responses in airway smooth muscle. Respir. Physiol. Neurobiol 137, 179–195 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

