Role of beta-adrenergic receptor subtypes in pig uterus contractility with inflammation
- PMID: 34075189
- PMCID: PMC8169833
- DOI: 10.1038/s41598-021-91184-5
Role of beta-adrenergic receptor subtypes in pig uterus contractility with inflammation
Abstract
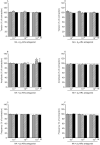
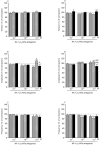
Uterine inflammation is a very common and serious condition in domestic animals. To development and progression of this pathology often lead disturbances in myometrial contractility. Participation of β1-, β2- and β3-adrenergic receptors (ARs) in noradrenaline (NA)-influenced contractility of the pig inflamed uterus was studied. The gilts of SAL- and E.coli-treated groups were administered saline or E.coli suspension into the uterine horns, respectively. Laparotomy was only done in the CON group. Compared to the period before NA administration, this neurotransmitter reduced the tension, amplitude and frequency in uterine strips of the CON and SAL groups. In the E.coli group, NA decreased the amplitude and frequency, and these parameters were lower than in other groups. In the CON, SAL and E.coli groups, β1- and β3-ARs antagonists in more cases did not significantly change and partly eliminated NA inhibitory effect on amplitude and frequency, as compared to NA action alone. In turn, β2-ARs antagonist completely abolished NA relaxatory effect on these parameters in three groups. Summarizing, NA decreases the contractile amplitude and frequency of pig inflamed uterus via all β-ARs subtypes, however, β2-ARs have the greatest importance. Given this, pharmacological modulation of particular β-ARs subtypes can be used to increase inflamed uterus contractility.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Effect of blocking of alpha1-adrenoreceptor isoforms on the noradrenaline-induced changes in contractility of inflamed pig uterus.PLoS One. 2023 Feb 17;18(2):e0280152. doi: 10.1371/journal.pone.0280152. eCollection 2023. PLoS One. 2023. PMID: 36800373 Free PMC article.
-
Roles of alpha-2-adrenergic receptor isoforms in inflamed pig uterus contractility in vitro.Theriogenology. 2022 Apr 15;183:41-52. doi: 10.1016/j.theriogenology.2022.01.025. Epub 2022 Feb 7. Theriogenology. 2022. PMID: 35202924
-
Expression of alpha and beta adrenergic receptors in the pig uterus during inflammation.Theriogenology. 2018 Oct 1;119:96-104. doi: 10.1016/j.theriogenology.2018.06.025. Epub 2018 Jun 28. Theriogenology. 2018. PMID: 29990768
-
Physiological consequences of beta-adrenergic receptor disruption.J Mol Med (Berl). 1998 Oct;76(11):764-72. doi: 10.1007/s001090050278. J Mol Med (Berl). 1998. PMID: 9826121 Review.
-
Contractility of the nonpregnant uterus: the follicular phase.Ann N Y Acad Sci. 2001 Sep;943:172-84. doi: 10.1111/j.1749-6632.2001.tb03801.x. Ann N Y Acad Sci. 2001. PMID: 11594539 Review.
Cited by
-
Investigation of the Role of Pituitary Adenylate Cyclase-Activating Peptide (PACAP) and Its Type 1 (PAC1) Receptor in Uterine Contractility during Endometritis in Pigs.Int J Mol Sci. 2022 May 13;23(10):5467. doi: 10.3390/ijms23105467. Int J Mol Sci. 2022. PMID: 35628275 Free PMC article.
-
Involvement of the calcitonin gene-related peptide system in the modulation of inflamed uterus contractile function in pigs.Sci Rep. 2022 Nov 9;12(1):19146. doi: 10.1038/s41598-022-23867-6. Sci Rep. 2022. PMID: 36352250 Free PMC article.
-
Effect of blocking of alpha1-adrenoreceptor isoforms on the noradrenaline-induced changes in contractility of inflamed pig uterus.PLoS One. 2023 Feb 17;18(2):e0280152. doi: 10.1371/journal.pone.0280152. eCollection 2023. PLoS One. 2023. PMID: 36800373 Free PMC article.
References
-
- Tummaruk P, Kesdangsakonwut S, Prapasarakul N, Kaeoket K. Endometritis in gilts: reproductive data, bacterial culture, histopathology, and infiltration of immune cells in the endometrium. Comp. Clin. Pathol. 2010;19:575–584. doi: 10.1007/s00580-009-0929-1. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

