Necroptosis molecular mechanisms: Recent findings regarding novel necroptosis regulators
- PMID: 34075202
- PMCID: PMC8166896
- DOI: 10.1038/s12276-021-00634-7
Necroptosis molecular mechanisms: Recent findings regarding novel necroptosis regulators
Abstract
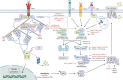
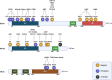
Necroptosis is a form of programmed necrosis that is mediated by various cytokines and pattern recognition receptors (PRRs). Cells dying by necroptosis show necrotic phenotypes, including swelling and membrane rupture, and release damage-associated molecular patterns (DAMPs), inflammatory cytokines, and chemokines, thereby mediating extreme inflammatory responses. Studies on gene knockout or necroptosis-specific inhibitor treatment in animal models have provided extensive evidence regarding the important roles of necroptosis in inflammatory diseases. The necroptosis signaling pathway is primarily modulated by activation of receptor-interacting protein kinase 3 (RIPK3), which phosphorylates mixed-lineage kinase domain-like protein (MLKL), mediating MLKL oligomerization. In the necroptosis process, these proteins are fine-tuned by posttranslational regulation via phosphorylation, ubiquitination, glycosylation, and protein-protein interactions. Herein, we review recent findings on the molecular regulatory mechanisms of necroptosis.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

