This is a preprint.
Fc-engineered antibody therapeutics with improved efficacy against COVID-19
- PMID: 34075373
- PMCID: PMC8168397
- DOI: 10.21203/rs.3.rs-555612/v1
Fc-engineered antibody therapeutics with improved efficacy against COVID-19
Update in
-
Fc-engineered antibody therapeutics with improved anti-SARS-CoV-2 efficacy.Nature. 2021 Nov;599(7885):465-470. doi: 10.1038/s41586-021-04017-w. Epub 2021 Sep 21. Nature. 2021. PMID: 34547765 Free PMC article.
Abstract
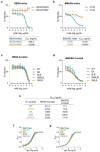
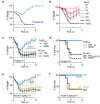
Monoclonal antibodies (mAbs) with neutralizing activity against SARS-CoV-2 have demonstrated clinical benefit in cases of mild to moderate SARS-CoV-2 infection, substantially reducing the risk for hospitalization and severe disease1-4. Treatment generally requires the administration of high doses of these mAbs with limited efficacy in preventing disease complications or mortality among hospitalized COVID-19 patients5. Here we report the development and evaluation of Fc-optimized anti-SARS-CoV-2 mAbs with superior potency to prevent or treat COVID-19 disease. In several animal models of COVID-19 disease6,7, we demonstrate that selective engagement of activating FcγRs results in improved efficacy in both preventing and treating disease-induced weight loss and mortality, significantly reducing the dose required to confer full protection upon SARS-CoV-2 challenge and treatment of pre-infected animals. Our results highlight the importance of FcγR pathways in driving antibody-mediated antiviral immunity, while excluding any pathogenic or disease-enhancing effects of FcγR engagement of anti-SARS-CoV-2 antibodies upon infection. These findings have important implications for the development of Fc-engineered mAbs with optimal Fc effector function and improved clinical efficacy against COVID-19 disease.
Conflict of interest statement
Figures



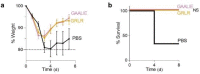




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

