Regulation of RNA polymerase II activity is essential for terminal erythroid maturation
- PMID: 34075391
- PMCID: PMC8569412
- DOI: 10.1182/blood.2020009903
Regulation of RNA polymerase II activity is essential for terminal erythroid maturation
Abstract
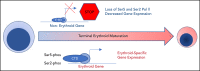
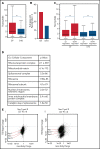
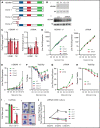
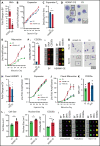
The terminal maturation of human erythroblasts requires significant changes in gene expression in the context of dramatic nuclear condensation. Defects in this process are associated with inherited anemias and myelodysplastic syndromes. The progressively dense appearance of the condensing nucleus in maturing erythroblasts led to the assumption that heterochromatin accumulation underlies this process, but despite extensive study, the precise mechanisms underlying this essential biologic process remain elusive. To delineate the epigenetic changes associated with the terminal maturation of human erythroblasts, we performed mass spectrometry of histone posttranslational modifications combined with chromatin immunoprecipitation coupled with high-throughput sequencing, Assay for Transposase Accessible Chromatin, and RNA sequencing. Our studies revealed that the terminal maturation of human erythroblasts is associated with a dramatic decline in histone marks associated with active transcription elongation, without accumulation of heterochromatin. Chromatin structure and gene expression were instead correlated with dynamic changes in occupancy of elongation competent RNA polymerase II, suggesting that terminal erythroid maturation is controlled largely at the level of transcription. We further demonstrate that RNA polymerase II "pausing" is highly correlated with transcriptional repression, with elongation competent RNA polymerase II becoming a scare resource in late-stage erythroblasts, allocated to erythroid-specific genes. Functional studies confirmed an essential role for maturation stage-specific regulation of RNA polymerase II activity during erythroid maturation and demonstrate a critical role for HEXIM1 in the regulation of gene expression and RNA polymerase II activity in maturing erythroblasts. Taken together, our findings reveal important insights into the mechanisms that regulate terminal erythroid maturation and provide a novel paradigm for understanding normal and perturbed erythropoiesis.
© 2021 by The American Society of Hematology.
Figures







Comment in
-
When Pol II sees red.Blood. 2021 Nov 4;138(18):1648-1649. doi: 10.1182/blood.2021012453. Blood. 2021. PMID: 34735000 Free PMC article. No abstract available.
References
-
- Della Porta MG, Travaglino E, Boveri E, et al. ; Rete Ematologica Lombarda (REL) Clinical Network . Minimal morphological criteria for defining bone marrow dysplasia: a basis for clinical implementation of WHO classification of myelodysplastic syndromes. Leukemia. 2015;29(1):66-75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

