The aryl hydrocarbon receptor in liver inflammation
- PMID: 34075438
- PMCID: PMC8443474
- DOI: 10.1007/s00281-021-00867-8
The aryl hydrocarbon receptor in liver inflammation
Abstract
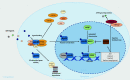
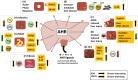
The aryl hydrocarbon receptor (AHR) is a ubiquitously expressed ligand-activated transcription factor with multifaceted physiological functions. In the immune system, AHR has been unequivocally identified as a key regulatory factor that can integrate environmental, dietary, or microbial signals into innate and adaptive immune responses. Correspondingly, AHR activity seems to be most important at barrier organs, such as the gut, skin, and lung. The liver is likewise prominently exposed to gut-derived dietary or microbial AHR ligands and, moreover, generates plenty of AHR ligands itself. Yet, surprisingly little is known about the role of AHR in the regulation of hepatic immune responses, which are normally biased towards tolerance, preventing harmful inflammation in response to innocuous stimuli. In this review, we summarize the current knowledge about the role of AHR in hepatic immune responses in the healthy liver as well as in inflammatory liver disease. Moreover, we discuss AHR as a potential therapeutic target in hepatic disorders, including autoimmune liver disease, liver fibrosis, and liver cancer.
Keywords: AHR ligands; Aryl hydrocarbon receptor; Hepatic immune response; Hepatic tolerance; Liver inflammation; Therapy.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

