The Evolution of Supply and Demand in Markets for Generic Drugs
- PMID: 34075623
- PMCID: PMC8452364
- DOI: 10.1111/1468-0009.12517
The Evolution of Supply and Demand in Markets for Generic Drugs
Abstract
Policy Points Much concern about generic drug markets has emerged in recent policy debates. Important changes in regulations, the structure of purchasing, and the length of the drug supply chain have affected generic drug markets. Effective price competition remains the rule in generic markets for large-selling drugs. Smaller markets and those for injectable products often have less price competition and are more susceptible to supply disruptions.
Context: The image of generic drugs as a commodity sold in competitive markets is an oversimplification, as evidenced by increasing accounts of price spikes, sustained high price-cost margins, and market disruptions. The mismatch between the canonical economic model of generic drug markets and reality motivated our empirical project.
Methods: To explore recent changes in those factors impacting the supply and demand for generic drugs, we studied, from a variety of sources, the data on price, competition, supply disruptions and recalls, changes to the supply chain, and buy-side concentration. We examined quarterly data through 2018 for a cohort of 77 molecules that lost patent protection during the so-called patent cliff between 2010 and 2013.
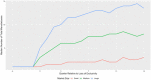
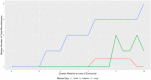
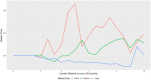
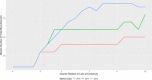
Findings: On the supply side, we found that for large-market oral solids, generic entry and price declines were consistent with past studies showing a significant number of market entrants and substantial reductions in the average price of a molecule. In smaller markets for oral solids and injectable products, we observed fewer entrants, higher rates of exit, smaller price reductions, and, in some cases, considerable price instability. The number of reported shortages increased across all generic market types over time, with the rate of shortage increases especially pronounced in markets for injectable products. The number of product recalls also rose over our study period. Although we did not estimate causal effects, we did find several changes in the market environment for generic drugs that may contribute to these phenomena. The demand side for generics has become more concentrated. Supply chains rely more on producers outside the United States (particularly from China and India). Contracting practices have undergone changes that may inhibit competition in product supply. FDA regulatory scruitiny has increased.
Conclusions: Competition in generic drug markets varies widely by market size and product form. Recent changes in demand-side market structure imply more downward pressure on prices stemming from buy-side concentration. The FDA's greater regulatory oversight puts upward pressure on costs, and the lengthening of the supply chain increases production uncertainty for producers. Demand and supply-side changes point to further market instabilities across all generic markets due to producers' changing economic position.
Keywords: drug prices; drug regulation; drug shortages; generic drugs.
© 2021 Milbank Memorial Fund.
Figures

References
-
- US Food & Drug Administration . Approved Drug Products with Therapeutic Equivalence Evaluations. 37th ed. Washington, DC: US Department of Health and Human Services; 2017.
-
- Association for Accessible Medicines . Generic drug access and savings in the U.S. 2017. https://accessible.meds.org/sites/default/files/2017‐07/2017‐AAM‐Access‐.... Accessed March 25, 2021.
-
- IQVIA . Medicine use and spending in the U.S. https://www.iqvia.com/insights/the‐iqvia‐institute/reports/medicine‐use‐.... May 2019. Accessed March 25, 2021.
-
- Scott Morton F, Kyle M. Markets for pharmaceutical products. In: Pauly M, McGuire T, Barros P, eds. Handbook of Health Economics. Vol. 2. Amsterdam, Netherlands: Elsevier (North Holland); 2012:763‐823.
-
- Berndt ER, Conti RM, Murphy SJ.The landscape of U.S. generic prescription drug markets: 2004–2016. NBER Working Paper 23640. July 2017. 10.3386/w23640. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

