Dwarf open reading frame (DWORF) is a direct activator of the sarcoplasmic reticulum calcium pump SERCA
- PMID: 34075877
- PMCID: PMC8203291
- DOI: 10.7554/eLife.65545
Dwarf open reading frame (DWORF) is a direct activator of the sarcoplasmic reticulum calcium pump SERCA
Abstract
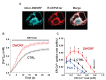
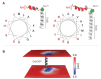
The sarco-plasmic reticulum calcium pump (SERCA) plays a critical role in the contraction-relaxation cycle of muscle. In cardiac muscle, SERCA is regulated by the inhibitor phospholamban. A new regulator, dwarf open reading frame (DWORF), has been reported to displace phospholamban from SERCA. Here, we show that DWORF is a direct activator of SERCA, increasing its turnover rate in the absence of phospholamban. Measurement of in-cell calcium dynamics supports this observation and demonstrates that DWORF increases SERCA-dependent calcium reuptake. These functional observations reveal opposing effects of DWORF activation and phospholamban inhibition of SERCA. To gain mechanistic insight into SERCA activation, fluorescence resonance energy transfer experiments revealed that DWORF has a higher affinity for SERCA in the presence of calcium. Molecular modeling and molecular dynamics simulations provide a model for DWORF activation of SERCA, where DWORF modulates the membrane bilayer and stabilizes the conformations of SERCA that predominate during elevated cytosolic calcium.
Keywords: biochemistry; calcium ATPase; cellular calcium dynamics; chemical biology; fluorescence resonance energy transfer; membrane reconstitution; molecular dynamics simulations; none.
© 2021, Fisher et al.
Conflict of interest statement
MF, EB, RA, EC, MP, MD, NR, LE, SR, AZ, HY No competing interests declared, ML Reviewing editor, eLife
Figures








References
-
- Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1-2:19–25. doi: 10.1016/j.softx.2015.06.001. - DOI
-
- Asahi M, McKenna E, Kurzydlowski K, Tada M, MacLennan DH. Physical interactions between phospholamban and sarco(endo)plasmic reticulum Ca2+-ATPases are dissociated by elevated Ca2+, but not by phospholamban phosphorylation, Vanadate, or thapsigargin, and are enhanced by ATP. Journal of Biological Chemistry. 2000;275:15034–15038. doi: 10.1074/jbc.275.20.15034. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

