TCF-ALP: a fluorescent probe for the selective detection of Staphylococcus bacteria and application in "smart" wound dressings
- PMID: 34075906
- PMCID: PMC8204156
- DOI: 10.1039/d0bm01918f
TCF-ALP: a fluorescent probe for the selective detection of Staphylococcus bacteria and application in "smart" wound dressings
Abstract
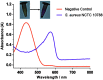
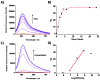
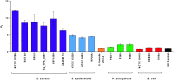
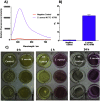
Alkaline phosphatase (ALP) is an important enzyme-based biomarker present in several bacterial species; however, it is currently undervalued as a strategy to detect pathogenic bacteria. Here, we explore our ALP-responsive colorimetric and fluorescent probe (TCF-ALP) for such applications. TCF-ALP displayed a colorimetric and fluorescence response towards Staphylococcus aureus (S. aureus), with a limit of detection of 3.7 × 106 CFU mL-1 after 24 h incubation. To our surprise, TCF-ALP proved selective towards Staphylococcus bacteria when compared with Enterococcus faecalis (E. faecalis), and Gram-negative P. aeruginosa and E. coli. Selectivity was also seen in clinically relevant S. aureus biofilms. Owing to the high prevalence and surface location of S. aureus in chronic wounds, TCF-ALP was subsequently encapsulated in polyvinyl alcohol (PVA)-based hydrogels as a proof-of-concept "smart" wound dressing. TCF-ALP hydrogels were capable of detecting S. aureus in planktonic and biofilm assays, and displayed a clear colour change from yellow to purple after 24 h incubation using ex vivo porcine skin models. Overall, TCF-ALP is a simple tool that requires no prior knowledge, training, or specialist equipment, and has the potential to overcome issues related to invasive swabbing and tissue biopsy methods. Thus, TCF-ALP could be used as a tool to monitor the early development of infection in a wound and allow for the rapid provision of appropriate treatment for Staphylococcal bacterial infections.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures


Similar articles
-
Prototype Development of the Intelligent Hydrogel Wound Dressing and Its Efficacy in the Detection of Model Pathogenic Wound Biofilms.ACS Appl Mater Interfaces. 2016 Jun 22;8(24):14909-19. doi: 10.1021/acsami.5b07372. Epub 2015 Oct 22. ACS Appl Mater Interfaces. 2016. PMID: 26492095
-
Long Wavelength TCF-Based Fluorescent Probe for the Detection of Alkaline Phosphatase in Live Cells.Front Chem. 2019 Apr 30;7:255. doi: 10.3389/fchem.2019.00255. eCollection 2019. Front Chem. 2019. PMID: 31119120 Free PMC article.
-
Cadexomer iodine provides superior efficacy against bacterial wound biofilms in vitro and in vivo.Wound Repair Regen. 2017 Jan;25(1):13-24. doi: 10.1111/wrr.12497. Epub 2016 Dec 5. Wound Repair Regen. 2017. PMID: 27859922
-
TCF-based fluorescent probe for monitoring superoxide anion produced in bacteria under chloramphenicol- and heat-induced stress.Chem Commun (Camb). 2022 Nov 24;58(94):13103-13106. doi: 10.1039/d2cc04662h. Chem Commun (Camb). 2022. PMID: 36342473
-
Controlling methicillin resistant Staphyloccocus aureus and Pseudomonas aeruginosa wound infections with a novel biomaterial.J Invest Surg. 2007 Jul-Aug;20(4):217-27. doi: 10.1080/10717540701481275. J Invest Surg. 2007. PMID: 17710602
Cited by
-
Advancements in antimicrobial nanoscale materials and self-assembling systems.Chem Soc Rev. 2022 Oct 17;51(20):8696-8755. doi: 10.1039/d1cs00915j. Chem Soc Rev. 2022. PMID: 36190355 Free PMC article. Review.
-
Novel Diagnostic Technologies and Therapeutic Approaches Targeting Chronic Wound Biofilms and Microbiota.Curr Dermatol Rep. 2022 Jun;11(2):60-72. doi: 10.1007/s13671-022-00354-9. Epub 2022 Mar 25. Curr Dermatol Rep. 2022. PMID: 37007641 Free PMC article.
-
Recent Progress in Identifying Bacteria with Fluorescent Probes.Molecules. 2022 Sep 29;27(19):6440. doi: 10.3390/molecules27196440. Molecules. 2022. PMID: 36234978 Free PMC article. Review.
-
Theoretical Investigation on the ESIPT Process and Detection Mechanism for Dual-Proton Type Fluorescent Probe.Int J Mol Sci. 2022 Feb 15;23(4):2132. doi: 10.3390/ijms23042132. Int J Mol Sci. 2022. PMID: 35216247 Free PMC article.
-
Intestinal Alkaline Phosphatase Activity and Efficiency Are Altered in Severe COVID-19 Patients.Gastro Hep Adv. 2023 Jul 17;2(7):911-917. doi: 10.1016/j.gastha.2023.07.003. eCollection 2023. Gastro Hep Adv. 2023. PMID: 39130768 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

