Group B Streptococcus cpsE Is Required for Serotype V Capsule Production and Aids in Biofilm Formation and Ascending Infection of the Reproductive Tract during Pregnancy
- PMID: 34076405
- PMCID: PMC8588567
- DOI: 10.1021/acsinfecdis.1c00182
Group B Streptococcus cpsE Is Required for Serotype V Capsule Production and Aids in Biofilm Formation and Ascending Infection of the Reproductive Tract during Pregnancy
Abstract
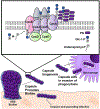
Group B Streptococcus (GBS) is an encapsulated Gram-positive pathogen that causes ascending infections of the reproductive tract during pregnancy. The capsule of this organism is a critical virulence factor that has been implicated in a variety of cellular processes to promote pathogenesis. Primarily comprised of carbohydrates, the GBS capsule and its synthesis is driven by the
Keywords: Streptococcus; capsule; carbohydrate; innate immunity; pathogenesis.
Figures




Similar articles
-
Mutations in pneumococcal cpsE generated via in vitro serial passaging reveal a potential mechanism of reduced encapsulation utilized by a conjunctival isolate.J Bacteriol. 2015 May;197(10):1781-91. doi: 10.1128/JB.02602-14. Epub 2015 Mar 16. J Bacteriol. 2015. PMID: 25777672 Free PMC article.
-
Streptococcus agalactiae npx Is Required for Survival in Human Placental Macrophages and Full Virulence in a Model of Ascending Vaginal Infection during Pregnancy.mBio. 2022 Dec 20;13(6):e0287022. doi: 10.1128/mbio.02870-22. Epub 2022 Nov 21. mBio. 2022. PMID: 36409087 Free PMC article.
-
Genomic analysis reveals the molecular basis for capsule loss in the group B Streptococcus population.PLoS One. 2015 May 6;10(5):e0125985. doi: 10.1371/journal.pone.0125985. eCollection 2015. PLoS One. 2015. PMID: 25946017 Free PMC article.
-
Biofilm formation by Streptococcus agalactiae: influence of environmental conditions and implicated virulence factors.Front Cell Infect Microbiol. 2015 Feb 4;5:6. doi: 10.3389/fcimb.2015.00006. eCollection 2015. Front Cell Infect Microbiol. 2015. PMID: 25699242 Free PMC article. Review.
-
Streptococcus agalactiae maternal colonization, antibiotic resistance and serotype profiles in Africa: a meta-analysis.Ann Clin Microbiol Antimicrob. 2019 Mar 28;18(1):14. doi: 10.1186/s12941-019-0313-1. Ann Clin Microbiol Antimicrob. 2019. PMID: 30922308 Free PMC article.
Cited by
-
A CRISPRi Library Screen in Group B Streptococcus Identifies Surface Immunogenic Protein (Sip) as a Mediator of Multiple Host Interactions.bioRxiv [Preprint]. 2024 Dec 7:2024.12.06.627252. doi: 10.1101/2024.12.06.627252. bioRxiv. 2024. Update in: Infect Immun. 2025 Apr 08;93(4):e0057324. doi: 10.1128/iai.00573-24. PMID: 39677656 Free PMC article. Updated. Preprint.
-
Virulence and pathogenicity of group B Streptococcus: Virulence factors and their roles in perinatal infection.Virulence. 2025 Dec;16(1):2451173. doi: 10.1080/21505594.2025.2451173. Epub 2025 Jan 23. Virulence. 2025. PMID: 39844743 Free PMC article. Review.
-
Identification of Glyoxalase A in Group B Streptococcus and its contribution to methylglyoxal tolerance and virulence.bioRxiv [Preprint]. 2024 Dec 19:2024.07.30.605887. doi: 10.1101/2024.07.30.605887. bioRxiv. 2024. Update in: Infect Immun. 2025 Apr 08;93(4):e0054024. doi: 10.1128/iai.00540-24. PMID: 39131367 Free PMC article. Updated. Preprint.
-
A CRISPRi library screen in group B Streptococcus identifies surface immunogenic protein (Sip) as a mediator of multiple host interactions.Infect Immun. 2025 Apr 8;93(4):e0057324. doi: 10.1128/iai.00573-24. Epub 2025 Mar 21. Infect Immun. 2025. PMID: 40116487 Free PMC article.
-
Re-framing the importance of Group B Streptococcus as a gut-resident pathobiont.Infect Immun. 2024 Sep 10;92(9):e0047823. doi: 10.1128/iai.00478-23. Epub 2024 Mar 4. Infect Immun. 2024. PMID: 38436256 Free PMC article. Review.
References
-
- Nanduri SA; Petit S; Smelser C; Apostol M; Alden NB; Harrison LH; Lynfield R; Vagnone PS; Burzlaff K; Spina NL; Dufort EM; Schaffner W; Thomas AR; Farley MM; Jain JH; Pondo T; McGee L; Beall BW; Schrag SJ Epidemiology of Invasive Early-Onset and Late-Onset Group B Streptococcal Disease in the United States, 2006 to 2015: Multistate Laboratory and Population-Based Surveillance. JAMA Pediatr. 2019, 173 (3), 224–233. 10.1001/jamapediatrics.2018.4826. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- UL1 TR000445/TR/NCATS NIH HHS/United States
- R01 HD090061/HD/NICHD NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- T32 AI112541/AI/NIAID NIH HHS/United States
- R01 AI134036/AI/NIAID NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- S10 RR027396/RR/NCRR NIH HHS/United States
- F32 HD100087/HD/NICHD NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
- K08 AI151100/AI/NIAID NIH HHS/United States
- U01 TR002398/TR/NCATS NIH HHS/United States
- R35 GM133602/GM/NIGMS NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- S10 RR026373/RR/NCRR NIH HHS/United States
- K12 HD000850/HD/NICHD NIH HHS/United States
- T32 HL007411/HL/NHLBI NIH HHS/United States
- I01 BX005352/BX/BLRD VA/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources

