Cooperativity of α-Synuclein Binding to Lipid Membranes
- PMID: 34076426
- PMCID: PMC8291482
- DOI: 10.1021/acschemneuro.1c00006
Cooperativity of α-Synuclein Binding to Lipid Membranes
Abstract
Cooperative binding is a key feature of metabolic pathways, signaling, and transport processes. It provides tight regulation over a narrow concentration interval of a ligand, thus enabling switching to be triggered by small concentration variations. The data presented in this work reveal strong positive cooperativity of α-synuclein binding to phospholipid membranes. Fluorescence cross-correlation spectroscopy, confocal microscopy, and cryo-TEM results show that in excess of vesicles α-synuclein does not distribute randomly but binds only to a fraction of all available vesicles. Furthermore, α-synuclein binding to a supported lipid bilayer observed with total internal reflection fluorescence microscopy displays a much steeper dependence of bound protein on total protein concentration than expected for independent binding. The same phenomenon was observed in the case of α-synuclein binding to unilamellar vesicles of sizes in the nm and μm range as well as to flat supported lipid bilayers, ruling out that nonuniform binding of the protein is governed by differences in membrane curvature. Positive cooperativity of α-synuclein binding to lipid membranes means that the affinity of the protein to a membrane is higher where there is already protein bound compared to a bare membrane. The phenomenon described in this work may have implications for α-synuclein function in synaptic transmission and other membrane remodeling events.
Keywords: Adair equation; Cooperative binding; fluorescence correlation spectroscopy; homotropic allostery; lipid membrane; α-synuclein.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




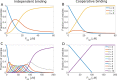
 × 106 M–1 where N is the total number of binding sites. Values
of the macroscopic binding constants for positively cooperative binding
to vesicles with two binding sites were K1 =
× 106 M–1 where N is the total number of binding sites. Values
of the macroscopic binding constants for positively cooperative binding
to vesicles with two binding sites were K1 =  × 106 M–1 and K2 =
4 × 106 M–1, assuming an average
affinity of 1 × 106 M-1 and a free
energy coupling of ΔΔG=-10
kJ mol-1. For the case of vesicles with 10 binding
sites and cooperative binding, the macroscopic binding constants were
K1 = 0.0745 M-1, K2 = 2.15
M-1, K3 = 81.4 M-1,
K4 = 3400 M-1, K5 = 1.5 ×
105 M-1, K6 = 6.7 × 106 M-1, K7 = 2.9 × 108 M-1, K8 = 1.2 × 1010 M-1, K9 = 4.7 × 1011 M-1 and K10 = 1.3 × 1013 M-1, assuming an average affinity of 1
× 106 M-1 and a free energy coupling
of ΔΔG=-10 kJ mol-1.
× 106 M–1 and K2 =
4 × 106 M–1, assuming an average
affinity of 1 × 106 M-1 and a free
energy coupling of ΔΔG=-10
kJ mol-1. For the case of vesicles with 10 binding
sites and cooperative binding, the macroscopic binding constants were
K1 = 0.0745 M-1, K2 = 2.15
M-1, K3 = 81.4 M-1,
K4 = 3400 M-1, K5 = 1.5 ×
105 M-1, K6 = 6.7 × 106 M-1, K7 = 2.9 × 108 M-1, K8 = 1.2 × 1010 M-1, K9 = 4.7 × 1011 M-1 and K10 = 1.3 × 1013 M-1, assuming an average affinity of 1
× 106 M-1 and a free energy coupling
of ΔΔG=-10 kJ mol-1.Similar articles
-
Binding of alpha-synuclein affects the lipid packing in bilayers of small vesicles.J Biol Chem. 2006 Apr 7;281(14):9251-9. doi: 10.1074/jbc.M512292200. Epub 2006 Feb 1. J Biol Chem. 2006. PMID: 16455667
-
Two different binding modes of α-synuclein to lipid vesicles depending on its aggregation state.Biophys J. 2012 Apr 4;102(7):1646-55. doi: 10.1016/j.bpj.2012.01.059. Epub 2012 Apr 3. Biophys J. 2012. PMID: 22500765 Free PMC article.
-
α-Synuclein senses lipid packing defects and induces lateral expansion of lipids leading to membrane remodeling.J Biol Chem. 2013 Jul 19;288(29):20883-20895. doi: 10.1074/jbc.M113.478297. Epub 2013 Jun 5. J Biol Chem. 2013. PMID: 23740253 Free PMC article.
-
Membrane interaction of α-synuclein in different aggregation states.J Parkinsons Dis. 2011;1(4):359-71. doi: 10.3233/JPD-2011-11067. J Parkinsons Dis. 2011. PMID: 23933657
-
Cooperativity in regulation of membrane protein function: phenomenological analysis of the effects of pH and phospholipids.Biophys Rev. 2023 Jul 18;15(4):721-731. doi: 10.1007/s12551-023-01095-0. eCollection 2023 Aug. Biophys Rev. 2023. PMID: 37681089 Free PMC article. Review.
Cited by
-
How important is the N-terminal acetylation of alpha-synuclein for its function and aggregation into amyloids?Front Neurosci. 2022 Nov 16;16:1003997. doi: 10.3389/fnins.2022.1003997. eCollection 2022. Front Neurosci. 2022. PMID: 36466161 Free PMC article. Review.
-
A Palette of Fluorescent Aβ42 Peptides Labelled at a Range of Surface-Exposed Sites.Int J Mol Sci. 2022 Jan 31;23(3):1655. doi: 10.3390/ijms23031655. Int J Mol Sci. 2022. PMID: 35163577 Free PMC article.
-
Relationship among α‑synuclein, aging and inflammation in Parkinson's disease (Review).Exp Ther Med. 2023 Nov 21;27(1):23. doi: 10.3892/etm.2023.12311. eCollection 2024 Jan. Exp Ther Med. 2023. PMID: 38125364 Free PMC article. Review.
-
Lipids and α-Synuclein: adding further variables to the equation.Front Mol Biosci. 2024 Aug 12;11:1455817. doi: 10.3389/fmolb.2024.1455817. eCollection 2024. Front Mol Biosci. 2024. PMID: 39188788 Free PMC article. Review.
-
α-Synuclein-induced deformation of small unilamellar vesicles.QRB Discov. 2022 Jul 25;3:e10. doi: 10.1017/qrd.2022.9. eCollection 2022. QRB Discov. 2022. PMID: 37529290 Free PMC article.
References
-
- Monod J. (1974) On chance and necessity. In Studies in the Philosophy of Biology, pp 357–375, Springer.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

