Nasal Swab Performance by Collection Timing, Procedure, and Method of Transport for Patients with SARS-CoV-2
- PMID: 34076471
- PMCID: PMC8373031
- DOI: 10.1128/JCM.00569-21
Nasal Swab Performance by Collection Timing, Procedure, and Method of Transport for Patients with SARS-CoV-2
Abstract
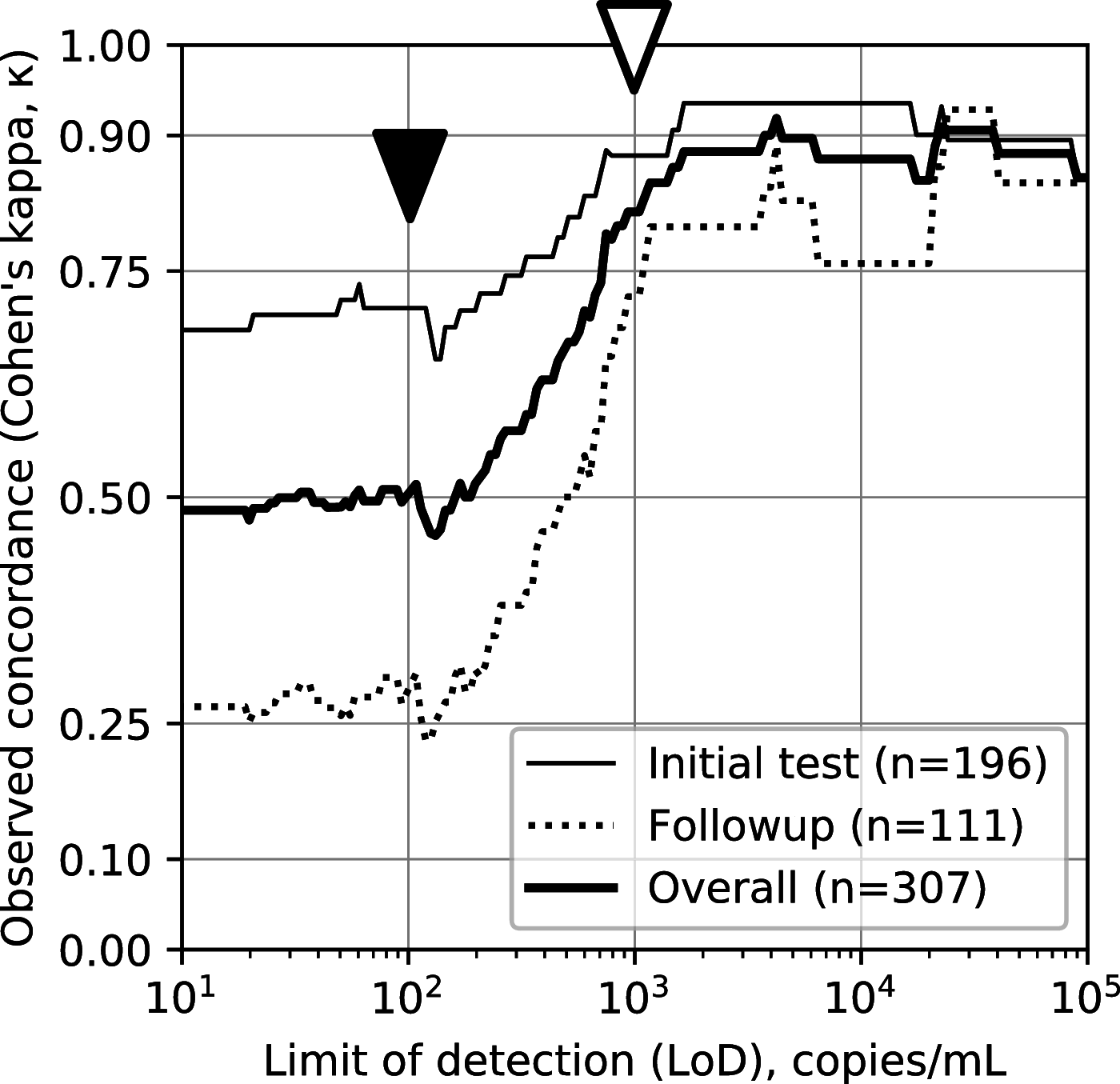
The urgent need for large-scale diagnostic testing for SARS-CoV-2 has prompted interest in sample collection methods of sufficient sensitivity to replace nasopharynx (NP) sampling. Nasal swab samples are an attractive alternative; however, previous studies have disagreed over how nasal sampling performs relative to NP sampling. Here, we compared nasal versus NP specimens collected by health care workers in a cohort of individuals clinically suspected of COVID-19 as well as SARS-CoV-2 reverse transcription (RT)-PCR-positive outpatients undergoing follow-up. We compared subjects being seen for initial evaluation versus follow-up, two different nasal swab collection protocols, and three different transport conditions, including traditional viral transport media (VTM) and dry swabs, on 307 total study participants. We compared categorical results and viral loads to those from standard NP swabs collected at the same time from the same patients. All testing was performed by RT-PCR on the Abbott SARS-CoV-2 RealTime emergency use authorization (EUA) (limit of detection [LoD], 100 copies viral genomic RNA/ml transport medium). We found low concordance overall, with Cohen's kappa (κ) of 0.49, with high concordance only for subjects with very high viral loads. We found medium concordance for testing at initial presentation (κ = 0.68) and very low concordance for follow-up testing (κ = 0.27). Finally, we show that previous reports of high concordance may have resulted from measurement using assays with sensitivity of ≥1,000 copies/ml. These findings suggest nasal-swab testing be used for situations in which viral load is expected to be high, as we demonstrate that nasal swab testing is likely to miss patients with low viral loads.
Keywords: COVID-19; NP swab; SARS-CoV-2; limit of detection; nasal swab.
Figures

Similar articles
-
Nasal-Swab Testing Misses Patients with Low SARS-CoV-2 Viral Loads.medRxiv [Preprint]. 2020 Jun 14:2020.06.12.20128736. doi: 10.1101/2020.06.12.20128736. medRxiv. 2020. PMID: 32587981 Free PMC article. Preprint.
-
Saliva is Comparable to Nasopharyngeal Swabs for Molecular Detection of SARS-CoV-2.Microbiol Spectr. 2021 Sep 3;9(1):e0016221. doi: 10.1128/Spectrum.00162-21. Epub 2021 Aug 18. Microbiol Spectr. 2021. PMID: 34406838 Free PMC article.
-
Concordance in RT-PCR detection of SARS-CoV-2 between samples preserved in viral and bacterial transport medium.J Virol Methods. 2022 Jun;304:114522. doi: 10.1016/j.jviromet.2022.114522. Epub 2022 Mar 9. J Virol Methods. 2022. PMID: 35278534 Free PMC article.
-
Comparison between Nasal and Nasopharyngeal Swabs for SARS-CoV-2 Rapid Antigen Detection in an Asymptomatic Population, and Direct Confirmation by RT-PCR from the Residual Buffer.Microbiol Spectr. 2022 Feb 23;10(1):e0245521. doi: 10.1128/spectrum.02455-21. Epub 2022 Feb 16. Microbiol Spectr. 2022. PMID: 35171010 Free PMC article.
-
Relative sensitivity of anterior nares and nasopharyngeal swabs for initial detection of SARS-CoV-2 in ambulatory patients: Rapid review and meta-analysis.PLoS One. 2021 Jul 20;16(7):e0254559. doi: 10.1371/journal.pone.0254559. eCollection 2021. PLoS One. 2021. PMID: 34283845 Free PMC article. Review.
Cited by
-
Cycle Threshold Values from Severe Acute Respiratory Syndrome Coronavirus-2 Reverse Transcription-Polymerase Chain Reaction Assays: Interpretation and Potential Use Cases.Clin Lab Med. 2022 Jun;42(2):237-248. doi: 10.1016/j.cll.2022.02.003. Epub 2022 Feb 21. Clin Lab Med. 2022. PMID: 35636824 Free PMC article. Review.
-
Diagnostic performance, user acceptability, and safety of unsupervised SARS-CoV-2 rapid antigen-detecting tests performed at home.Int J Infect Dis. 2022 Mar;116:358-364. doi: 10.1016/j.ijid.2022.01.019. Epub 2022 Jan 14. Int J Infect Dis. 2022. PMID: 35038598 Free PMC article.
-
ct2vl: A Robust Public Resource for Converting SARS-CoV-2 Ct Values to Viral Loads.Viruses. 2024 Jun 30;16(7):1057. doi: 10.3390/v16071057. Viruses. 2024. PMID: 39066220 Free PMC article.
-
Contrasting Effects of SARS-CoV-2 Vaccination vs. Infection on Antibody and TCR Repertoires.bioRxiv [Preprint]. 2023 Sep 11:2023.09.08.556703. doi: 10.1101/2023.09.08.556703. bioRxiv. 2023. PMID: 39829775 Free PMC article. Preprint.
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
References
-
- Yang Y, Yang M, Shen C, Wang F, Yuan J, Li J, Zhang M, Wang Z, Xing L, Wei J, Peng L, Wong G, Zheng H, Wu W, Liao M, Feng K, Li J, Yang Q, Zhao J, Zhang Z, Liu L, Liu Y. 2020. Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. medRxiv 10.1101/2020.02.11.20021493. - DOI
-
- Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C. 2020. Virological assessment of hospitalized patients with COVID-2019. Nature 581:465–469. 10.1038/s41586-020-2196-x. - DOI - PubMed
-
- Wyllie AL, Fournier J, Casanovas-Massana A, Campbell M, Tokuyama M, Vijayakumar P, Warren JL, Geng B, Muenker MC, Moore AJ, Vogels CBF, Petrone ME, Ott IM, Lu P, Venkataraman A, Lu-Culligan A, Klein J, Earnest R, Simonov M, Datta R, Handoko R, Naushad N, Sewanan LR, Valdez J, White ER, Lapidus S, Kalinich CC, Jiang X, Kim DJ, Kudo E, Linehan M, Mao T, Moriyama M, Oh JE, Park A, Silva J, Song E, Takahashi T, Taura M, Weizman O-E, Wong P, Yang Y, Bermejo S, Odio CD, Omer SB, Dela Cruz CS, Farhadian S, Martinello RA, Iwasaki A, Grubaugh ND, Ko AI. 2020. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N Engl J Med 383:1283–1286. 10.1056/NEJMc2016359. - DOI - PMC - PubMed
-
- CDC. 2020. Interim guidelines for collecting and handling of clinical specimens for COVID-19 testing. https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specim.... Accessed 10 June 2020.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

