Intranasal Nanoparticle Vaccination Elicits a Persistent, Polyfunctional CD4 T Cell Response in the Murine Lung Specific for a Highly Conserved Influenza Virus Antigen That Is Sufficient To Mediate Protection from Influenza Virus Challenge
- PMID: 34076479
- PMCID: PMC8373229
- DOI: 10.1128/JVI.00841-21
Intranasal Nanoparticle Vaccination Elicits a Persistent, Polyfunctional CD4 T Cell Response in the Murine Lung Specific for a Highly Conserved Influenza Virus Antigen That Is Sufficient To Mediate Protection from Influenza Virus Challenge
Abstract
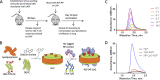
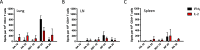
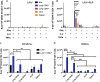
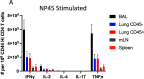
Lung-localized CD4 T cells play a critical role in the control of influenza virus infection and can provide broadly protective immunity. However, current influenza vaccination strategies primarily target influenza hemagglutinin (HA) and are administered peripherally to induce neutralizing antibodies. We have used an intranasal vaccination strategy targeting the highly conserved influenza nucleoprotein (NP) to elicit broadly protective lung-localized CD4 T cell responses. The vaccine platform consists of a self-assembling nanolipoprotein particle (NLP) linked to NP with an adjuvant. We have evaluated the functionality, in vivo localization, and persistence of the T cells elicited. Our study revealed that intranasal vaccination elicits a polyfunctional subset of lung-localized CD4 T cells that persist long term. A subset of these lung CD4 T cells localize to the airway, where they can act as early responders following encounter with cognate antigen. Polyfunctional CD4 T cells isolated from airway and lung tissue produce significantly more effector cytokines IFN-γ and TNF-α, as well as cytotoxic functionality. When adoptively transferred to naive recipients, CD4 T cells from NLP:NP-immunized lung were sufficient to mediate 100% survival from lethal challenge with H1N1 influenza virus. IMPORTANCE Exploiting new, more efficacious strategies to potentiate influenza virus-specific immune responses is important, particularly for at-risk populations. We have demonstrated the promise of direct intranasal protein vaccination to establish long-lived immunity in the lung with CD4 T cells that possess features and positioning in the lung that are associated with both immediate and long-term immunity, as well as demonstrating direct protective potential.
Keywords: CD4 T cells; Trm; airway T cells; influenza A; influenza virus challenge; lung parenchyma vasculature; mucosal immunology; polyfunctional T cells; rational vaccine design; tissue resident memory.
Figures







Similar articles
-
Broadly Protective CD8+ T Cell Immunity to Highly Conserved Epitopes Elicited by Heat Shock Protein gp96-Adjuvanted Influenza Monovalent Split Vaccine.J Virol. 2021 May 24;95(12):e00507-21. doi: 10.1128/JVI.00507-21. Print 2021 May 24. J Virol. 2021. PMID: 33827939 Free PMC article.
-
CCR2 Regulates Vaccine-Induced Mucosal T-Cell Memory to Influenza A Virus.J Virol. 2021 Jul 12;95(15):e0053021. doi: 10.1128/JVI.00530-21. Epub 2021 Jul 12. J Virol. 2021. PMID: 33952647 Free PMC article.
-
Cross-Protective Potential and Protection-Relevant Immune Mechanisms of Whole Inactivated Influenza Virus Vaccines Are Determined by Adjuvants and Route of Immunization.Front Immunol. 2019 Mar 29;10:646. doi: 10.3389/fimmu.2019.00646. eCollection 2019. Front Immunol. 2019. PMID: 30984200 Free PMC article.
-
Potentiating Lung Mucosal Immunity Through Intranasal Vaccination.Front Immunol. 2021 Dec 14;12:808527. doi: 10.3389/fimmu.2021.808527. eCollection 2021. Front Immunol. 2021. PMID: 34970279 Free PMC article. Review.
-
Distinct and complementary roles of CD4 T cells in protective immunity to influenza virus.Curr Opin Immunol. 2018 Aug;53:13-21. doi: 10.1016/j.coi.2018.03.019. Epub 2018 Apr 2. Curr Opin Immunol. 2018. PMID: 29621639 Free PMC article. Review.
Cited by
-
Location versus ID: what matters to lung-resident memory T cells?Front Immunol. 2024 Feb 5;15:1355910. doi: 10.3389/fimmu.2024.1355910. eCollection 2024. Front Immunol. 2024. PMID: 38375476 Free PMC article.
-
Relative deficiency in interferon-γ-secreting CD4+ T cells is strongly associated with poorer COVID-19 vaccination responses in older adults.Aging Cell. 2024 Apr;23(4):e14099. doi: 10.1111/acel.14099. Epub 2024 Feb 5. Aging Cell. 2024. PMID: 38317404 Free PMC article.
-
Targeting murine metastatic cancers with cholera toxin A1-adjuvanted peptide vaccines.Hum Vaccin Immunother. 2025 Dec;21(1):2455240. doi: 10.1080/21645515.2025.2455240. Epub 2025 Jan 23. Hum Vaccin Immunother. 2025. PMID: 39848921 Free PMC article.
-
Recent Advances in Inhaled Nanoformulations of Vaccines and Therapeutics Targeting Respiratory Viral Infections.Pharm Res. 2023 May;40(5):1015-1036. doi: 10.1007/s11095-023-03520-1. Epub 2023 Apr 25. Pharm Res. 2023. PMID: 37186073 Free PMC article. Review.
-
The unfulfilled potential of mucosal immunization.J Allergy Clin Immunol. 2022 Jul;150(1):1-11. doi: 10.1016/j.jaci.2022.05.002. Epub 2022 May 13. J Allergy Clin Immunol. 2022. PMID: 35569567 Free PMC article. Review.
References
-
- Rolfes MA, Foppa IM, Garg S, Flannery B, Brammer L, Singleton JA, Burns E, Jernigan D, Olsen SJ, Bresee J, Reed C. 2018. Annual estimates of the burden of seasonal influenza in the United States: a tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses 12:132–137. 10.1111/irv.12486. - DOI - PMC - PubMed
-
- Reed C, Chaves SS, Daily Kirley P, Emerson R, Aragon D, Hancock EB, Butler L, Baumbach J, Hollick G, Bennett NM, Laidler MR, Thomas A, Meltzer MI, Finelli L. 2015. Estimating influenza disease burden from population-based surveillance data in the United States. PLoS One 10:e0118369. 10.1371/journal.pone.0118369. - DOI - PMC - PubMed
-
- Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Cohen C, Gran JM, Schanzer D, Cowling BJ, Wu P, Kyncl J, Ang LW, Park M, Redlberger-Fritz M, Yu H, Espenhain L, Krishnan A, Emukule G, van Asten L, Pereira da Silva S, Aungkulanon S, Buchholz U, Widdowson M-A, Bresee JS, Azziz-Baumgartner E, Cheng P-Y, Dawood F, Foppa I, Olsen S, Haber M, Jeffers C, MacIntyre CR, Newall AT, Wood JG, Kundi M, Popow-Kraupp T, Ahmed M, Rahman M, Marinho F, Sotomayor Proschle CV, Vergara Mallegas N, Luzhao F, Sa L, Barbosa-Ramírez J, Sanchez DM, Gomez LA, Vargas XB, Acosta Herrera a, Llanés MJ, et al.. 2018. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 391:1285–1300. 10.1016/S0140-6736(17)33293-2. - DOI - PMC - PubMed
-
- Richards KA, Moritzky S, Shannon I, Fitzgerald T, Yang H, Branche A, Topham DJ, Treanor JJ, Nayak J, Sant AJ. 2020. Recombinant HA-based vaccine outperforms split and subunit vaccines in elicitation of influenza-specific CD4 T cells and CD4 T cell-dependent antibody responses in humans. NPJ Vaccines 5:77. 10.1038/s41541-020-00227-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

