African-Lineage Zika Virus Replication Dynamics and Maternal-Fetal Interface Infection in Pregnant Rhesus Macaques
- PMID: 34076485
- PMCID: PMC8312872
- DOI: 10.1128/JVI.02220-20
African-Lineage Zika Virus Replication Dynamics and Maternal-Fetal Interface Infection in Pregnant Rhesus Macaques
Abstract
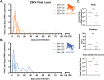
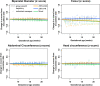
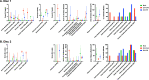
Following the Zika virus (ZIKV) outbreak in the Americas, ZIKV was causally associated with microcephaly and a range of neurological and developmental symptoms, termed congenital Zika syndrome (CZS). The viruses responsible for this outbreak belonged to the Asian lineage of ZIKV. However, in vitro and in vivo studies assessing the pathogenesis of African-lineage ZIKV demonstrated that African-lineage isolates often replicated to high titers and caused more-severe pathology than Asian-lineage isolates. To date, the pathogenesis of African-lineage ZIKV in a translational model, particularly during pregnancy, has not been rigorously characterized. Here, we infected four pregnant rhesus macaques with a low-passage-number strain of African-lineage ZIKV and compared its pathogenesis to those for a cohort of four pregnant rhesus macaques infected with an Asian-lineage isolate and a cohort of mock-inoculated controls. The viral replication kinetics for the two experimental groups were not significantly different, and both groups developed robust neutralizing antibody titers above levels considered to be protective. There was no evidence of significant fetal head growth restriction or gross fetal harm at delivery (1 to 1.5 weeks prior to full term) in either group. However, a significantly higher burden of ZIKV viral RNA (vRNA) was found in the maternal-fetal interface tissues of the macaques exposed to an African-lineage isolate. Our findings suggest that ZIKV of any genetic lineage poses a threat to pregnant individuals and their infants. IMPORTANCE ZIKV was first identified in 1947 in Africa, but most of our knowledge of ZIKV is based on studies of the distinct Asian genetic lineage, which caused the outbreak in the Americas in 2015 to 2016. In its most recent update, the WHO stated that improved understanding of African-lineage ZIKV pathogenesis during pregnancy must be a priority. The recent detection of African-lineage isolates in Brazil underscores the need to understand the impact of these viruses. Here, we provide the first comprehensive assessment of African-lineage ZIKV infection during pregnancy in a translational nonhuman primate model. We show that African-lineage isolates replicate with kinetics similar to those of Asian-lineage isolates and can infect the placenta. However, there was no evidence of more-severe outcomes with African-lineage isolates. Our results highlight both the threat that African-lineage ZIKV poses to pregnant individuals and their infants and the need for epidemiological and translational in vivo studies with African-lineage ZIKV.
Keywords: ZIKV; Zika virus; arbovirus; congenital Zika syndrome; flavivirus; macaque; pregnant.
Figures




References
-
- Grubaugh ND, Saraf S, Gangavarapu K, Watts A, Tan AL, Oidtman RJ, Ladner JT, Oliveira G, Matteson NL, Kraemer MUG, Vogels CBF, Hentoff A, Bhatia D, Stanek D, Scott B, Landis V, Stryker I, Cone MR, Kopp EW, Cannons AC, Heberlein-Larson L, White S, Gillis LD, Ricciardi MJ, Kwal J, Lichtenberger PK, Magnani DM, Watkins DI, Palacios G, Hamer DH, GeoSentinel Surveillance Network, Gardner LM, Perkins TA, Baele G, Khan K, Morrison A, Isern S, Michael SF, Andersen KG. 2019. Travel surveillance and genomics uncover a hidden Zika outbreak during the waning epidemic. Cell 178:1057–1071.e11. 10.1016/j.cell.2019.07.018. - DOI - PMC - PubMed
-
- Yuan L, Huang XY, Liu ZY, Zhang F, Zhu XL, Yu JY, Ji X, Xu YP, Li G, Li C, Wang HJ, Deng YQ, Wu M, Cheng ML, Ye Q, Xie DY, Li XF, Wang X, Shi W, Hu B, Shi PY, Xu Z, Qin CF. 2017. A single mutation in the prM protein of Zika virus contributes to fetal microcephaly. Science 358:933–936. 10.1126/science.aam7120. - DOI - PubMed
-
- Simonin Y, Loustalot F, Desmetz C, Foulongne V, Constant O, Fournier-Wirth C, Leon F, Molès JP, Goubaud A, Lemaitre JM, Maquart M, Leparc-Goffart I, Briant L, Nagot N, Van de Perre P, Salinas S. 2016. Zika virus strains potentially display different infectious profiles in human neural cells. EBioMedicine 12:161–169. 10.1016/j.ebiom.2016.09.020. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

