Invasive Haemophilus influenzae Infections after 3 Decades of Hib Protein Conjugate Vaccine Use
- PMID: 34076491
- PMCID: PMC8262803
- DOI: 10.1128/CMR.00028-21
Invasive Haemophilus influenzae Infections after 3 Decades of Hib Protein Conjugate Vaccine Use
Abstract
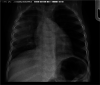
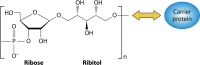
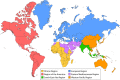
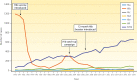
Haemophilus influenzae serotype b (Hib) was previously the most common cause of bacterial meningitis and an important etiologic agent of pneumonia in children aged <5 years. Its major virulence factor is the polyribosyl ribitol phosphate (PRP) polysaccharide capsule. In the 1980s, PRP-protein conjugate Hib vaccines were developed and are now included in almost all national immunization programs, achieving a sustained decline in invasive Hib infections. However, invasive Hib disease has not yet been eliminated in countries with low vaccine coverage, and sporadic outbreaks of Hib infection still occur occasionally in countries with high vaccine coverage. Over the past 2 decades, other capsulated serotypes have been recognized increasingly as causing invasive infections. H. influenzae serotype a (Hia) is now a major cause of invasive infection in Indigenous communities of North America, prompting a possible requirement for an Hia conjugate vaccine. H. influenzae serotypes e and f are now more common than serotype b in Europe. Significant year-to-year increases in nontypeable H. influenzae invasive infections have occurred in many regions of the world. Invasive H. influenzae infections are now seen predominantly in patients at the extremes of life and those with underlying comorbidities. This review provides a comprehensive and critical overview of the current global epidemiology of invasive H. influenzae infections in different geographic regions of the world. It discusses those now at risk of invasive Hib disease, describes the emergence of other severe invasive H. influenzae infections, and emphasizes the importance of long-term, comprehensive, clinical and microbiologic surveillance to monitor a vaccine's impact.
Keywords: Haemophilus influenzae; Hib; conjugate vaccine; epidemiology; immunization; nontypeable H. influenzae.
Figures
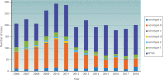
References
-
- Moxon ER. 1995. Haemophilus influenzae, p 2039–2050. In Mandell GL, Bennett JE, Dolin R (ed), Mandell, Douglas and Bennett's principles and practice of infectious disease, 4th ed, vol 2. Churchill Livingstone, New York, NY.
-
- Käyhty H, Karanko V, Peltola H, Mäkelä PH. 1984. Serum antibodies after vaccination with Haemophilus influenzae type b capsular polysaccharide and responses to reimmunization: no evidence of immunologic tolerance or memory. Pediatrics 74:857–865. - PubMed
-
- Peltola H. 2000. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin Microbiol Rev 13:302–317. 10.1128/CMR.13.2.302. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

