Large Clostridial Toxins: Mechanisms and Roles in Disease
- PMID: 34076506
- PMCID: PMC8483668
- DOI: 10.1128/MMBR.00064-21
Large Clostridial Toxins: Mechanisms and Roles in Disease
Abstract
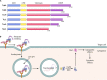
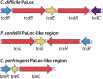
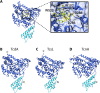
Large clostridial toxins (LCTs) are a family of bacterial exotoxins that infiltrate and destroy target cells. Members of the LCT family include Clostridioides difficile toxins TcdA and TcdB, Paeniclostridium sordellii toxins TcsL and TcsH, Clostridium novyi toxin TcnA, and Clostridium perfringens toxin TpeL. Since the 19th century, LCT-secreting bacteria have been isolated from the blood, organs, and wounds of diseased individuals, and LCTs have been implicated as the primary virulence factors in a variety of infections, including C. difficile infection and some cases of wound-associated gas gangrene. Clostridia express and secrete LCTs in response to various physiological signals. LCTs invade host cells by binding specific cell surface receptors, ultimately leading to internalization into acidified vesicles. Acidic pH promotes conformational changes within LCTs, which culminates in translocation of the N-terminal glycosyltransferase and cysteine protease domain across the endosomal membrane and into the cytosol, leading first to cytopathic effects and later to cytotoxic effects. The focus of this review is on the role of LCTs in infection and disease, the mechanism of LCT intoxication, with emphasis on recent structural work and toxin subtyping analysis, and the genomic discovery and characterization of LCT homologues. We provide a comprehensive review of these topics and offer our perspective on emerging questions and future research directions for this enigmatic family of toxins.
Keywords: Clostridium difficile; large clostridial toxin; toxin; toxin-mediated diseases; toxin-receptor interaction.
Figures



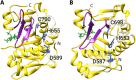
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

