pH-Dependent Flavin Adenine Dinucleotide and Nicotinamide Adenine Dinucleotide Ultraviolet Resonance Raman (UVRR) Spectra at Intracellular Concentration
- PMID: 34076541
- PMCID: PMC8320563
- DOI: 10.1177/00037028211025575
pH-Dependent Flavin Adenine Dinucleotide and Nicotinamide Adenine Dinucleotide Ultraviolet Resonance Raman (UVRR) Spectra at Intracellular Concentration
Abstract
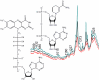
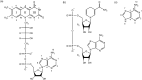
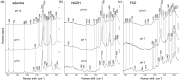
The ultraviolet resonance Raman spectra of the adenine-containing enzymatic redox cofactors nicotinamide adenine dinucleotide and flavin adenine dinucleotide in aqueous solution of physiological concentration are compared with the aim of distinguishing between them and their building block adenine in potential co-occurrence in biological materials. At an excitation wavelength of 266 nm, the spectra are dominated by the strong resonant contribution from adenine; nevertheless, bands assigned to vibrational modes of the nicotinamide and the flavin unit are found to appear at similar signal strength. Comparison of spectra measured at pH 7 with data obtained pH 10 and pH 3 shows characteristic changes when pH is increased or lowered, mainly due to deprotonation of the flavin and nicotinamide moieties, and protonation of the adenine, respectively.
Keywords: FAD; NADH; UVRR; Ultraviolet resonance Raman; adenine; flavin adenine dinucleotide; nicotinamide adenine dinucleotide; pH dependence; protonation.
Conflict of interest statement
Figures

Similar articles
-
Extracellular pH affects the fluorescence lifetimes of metabolic co-factors.J Biomed Opt. 2021 May;26(5):056502. doi: 10.1117/1.JBO.26.5.056502. J Biomed Opt. 2021. PMID: 34032035 Free PMC article.
-
In vivo monitoring the changes of interstitial pH and FAD/NADH ratio by fluorescence spectroscopy in healing skin wounds.Photochem Photobiol. 2006 May-Jun;82(3):793-7. doi: 10.1562/2005-09-08-RA-678. Photochem Photobiol. 2006. PMID: 16435883
-
Calculation of the Geometries and Infrared Spectra of the Stacked Cofactor Flavin Adenine Dinucleotide (FAD) as the Prerequisite for Studies of Light-Triggered Proton and Electron Transfer.Biomolecules. 2020 Apr 9;10(4):573. doi: 10.3390/biom10040573. Biomolecules. 2020. PMID: 32283685 Free PMC article.
-
Label-Free Optical Metabolic Imaging in Cells and Tissues.Annu Rev Biomed Eng. 2023 Jun 8;25:413-443. doi: 10.1146/annurev-bioeng-071516-044730. Epub 2023 Apr 27. Annu Rev Biomed Eng. 2023. PMID: 37104650 Free PMC article. Review.
-
Mitochondrial transport and metabolism of the vitamin B-derived cofactors thiamine pyrophosphate, coenzyme A, FAD and NAD+ , and related diseases: A review.IUBMB Life. 2022 Jul;74(7):592-617. doi: 10.1002/iub.2612. Epub 2022 Mar 18. IUBMB Life. 2022. PMID: 35304818 Free PMC article. Review.
Cited by
-
UV-SERS monitoring of plasmonic photodegradation of biomolecules on aluminum platforms decorated with rhodium nanoparticles.Nanoscale Adv. 2025 Jul 7;7(17):5212-5220. doi: 10.1039/d5na00486a. eCollection 2025 Aug 19. Nanoscale Adv. 2025. PMID: 40697506 Free PMC article.
References
-
- Stocchi V., Cucchiarini L., Magnani M., Fornaini G. “Adenine and Pyridine Nucleotides in the Erythrocyte of Different Mammalian Species”. Biochem. Int. 1987. 14(6): 1043–1053. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

