Pharmacokinetics of darunavir and cobicistat in pregnant and postpartum women with HIV
- PMID: 34076612
- PMCID: PMC8173003
- DOI: 10.1097/QAD.0000000000002857
Pharmacokinetics of darunavir and cobicistat in pregnant and postpartum women with HIV
Abstract
Objective: To evaluate darunavir and cobicistat pharmacokinetics during pregnancy compared with postpartum and in infant washout samples after delivery.
Design: Nonrandomized, open-label, parallel-group, multicenter phase-IV prospective study of darunavir and cobicistat pharmacokinetics in pregnant women with HIV and their children in the United States.
Methods: Intensive steady-state 24-h pharmacokinetic profiles were performed after administration of 800 mg of darunavir and 150 mg of cobicistat orally in fixed dose combination once-daily during the second trimester, third trimester, and postpartum. Infant washout samples were collected after birth. Darunavir and cobicistat were measured in plasma by validated HPLC-UV and liquid chromatography with tandem mass spectrometry detection (LC-MS)/MS assays, respectively. A two-tailed Wilcoxon signed-rank test (α = 0.10) was employed for paired within-participant comparisons.
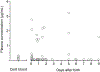
Results: A total of 29 pregnant women receiving darunavir and cobicistat once-daily enrolled in the study. Compared with paired postpartum data, darunavir AUC0--24 was 53% lower in the second trimester [n = 12, P = 0.0024, geometric mean of ratio (GMR)=0.47, 90% confidence interval (CI) 0.33 - 0.68] and 56% lower in the third trimester (n = 18, P < 0.0001, GMR = 0.44, 90% CI 0.36 - 0.54), whereas cobicistat AUC0--24 was 50% lower in the second trimester (n = 12, P = 0.0024, GMR = 0.50, 90% CI 0.36-0.69) and 56% lower in the third trimester (n = 18, P < 0.0001, GMR = 0.44, 90% CI 0.35-0.55). Placental transfer of darunavir and cobicistat was limited.
Conclusion: Standard darunavir/cobicistat dosing during pregnancy results in significantly lower exposure during pregnancy, which may increase the risk of virologic failure and perinatal transmission.
Copyright © 2021 Wolters Kluwer Health, Inc. All rights reserved.
Figures
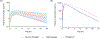
References
-
- Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission [September 30, 2019]; Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf.
-
- Mirochnick M, Capparelli E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet 2004; 43(15):1071–1087. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

