New insights into glial scar formation after spinal cord injury
- PMID: 34076775
- PMCID: PMC8975767
- DOI: 10.1007/s00441-021-03477-w
New insights into glial scar formation after spinal cord injury
Abstract
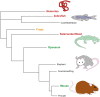
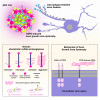
Severe spinal cord injury causes permanent loss of function and sensation throughout the body. The trauma causes a multifaceted torrent of pathophysiological processes which ultimately act to form a complex structure, permanently remodeling the cellular architecture and extracellular matrix. This structure is traditionally termed the glial/fibrotic scar. Similar cellular formations occur following stroke, infection, and neurodegenerative diseases of the central nervous system (CNS) signifying their fundamental importance to preservation of function. It is increasingly recognized that the scar performs multiple roles affecting recovery following traumatic injury. Innovative research into the properties of this structure is imperative to the development of treatment strategies to recover motor function and sensation following CNS trauma. In this review, we summarize how the regeneration potential of the CNS alters across phyla and age through formation of scar-like structures. We describe how new insights from next-generation sequencing technologies have yielded a more complex portrait of the molecular mechanisms governing the astrocyte, microglial, and neuronal responses to injury and development, especially of the glial component of the scar. Finally, we discuss possible combinatorial therapeutic approaches centering on scar modulation to restore function after severe CNS injury.
Keywords: Chondroitin sulfate proteoglycans; Glia; Glial scar; Glial scar formation; Single-cell RNA sequencing; Spinal cord injury.
© 2021. The Author(s).
Conflict of interest statement
A patent (9,937,242) filed by CWRU has been granted for ISP and licensed to NervGen Pharma Corp. The remaining authors declare no competing conflicts of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

