Opioid-Sparing Multimodal Analgesia Protocol for Lumpectomy Patients Results in Superior Postoperative Pain Control
- PMID: 34076809
- PMCID: PMC8170864
- DOI: 10.1245/s10434-021-09963-3
Opioid-Sparing Multimodal Analgesia Protocol for Lumpectomy Patients Results in Superior Postoperative Pain Control
Abstract
Background: We sought to determine if lumpectomy patients who received perioperative opioid-sparing multimodal analgesia reported less pain when compared with those who received traditional opioid-based care.
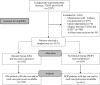
Study design: A prospective cohort of patients undergoing lumpectomy who received an opioid-sparing multimodal analgesia protocol [no opioids group (NOP)] was compared with a large cohort of patients who received traditional care [opioids group (OG)]. In-hospital and discharge opioids were compared using oral morphine equivalents (OMEs). Postoperative day one and week one pain scores were compared using the Kruskal-Wallis test.
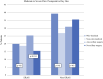
Results: Overall, 1153 patients underwent lumpectomy: 634 patients received the protocol (NOP), and 519 patients did not (OG). Median pain scores were significantly lower in the NOP cohort when compared with the OG cohort the day after surgery (2 vs. 0, p < 0.001) and the week after surgery (1 vs. 0, p < 0.001). NOP patients were significantly less likely to report severe pain (7-10 on a 10-point scale) the day after surgery compared with OG patients (15.7% vs. 6.9%, p = 0.004). Patients in the NOP cohort were discharged with a median of zero OMEs (range 0-150), while patients in the OG were discharged with a median of 90 OMEs (range 0-360; p < 0.001).
Conclusion: Implementation of an opioid-sparing multimodal analgesia protocol for lumpectomy patients resulted in superior pain control without a routine opioid prescription. Surgeons can improve their own patients' outcomes while addressing the larger societal issue of the opioid crisis by adopting similar protocols that decrease the quantity of opioids available for diversion.
© 2021. Society of Surgical Oncology.
Conflict of interest statement
Patrick Borgen, Kristin E. Rojas and Donna-Marie Manasseh have received speaker’s honoraria from Pacira Pharmaceuticals, Inc. Claudya Morin, Yamini Patel, Munazza Javid, Sarah E. Tevis, Thais Fortes, Peter Flom, and Charusheela Andaz report no relevant commercial, financial, consultant, or institutional conflicts of interest.
Figures

References
-
- American Cancer Society . Breast cancer facts and figures 2019–2020. Atlanta: American Cancer Society, Inc.; 2019.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

