Chronic lithium treatment alters the excitatory/ inhibitory balance of synaptic networks and reduces mGluR5-PKC signalling in mouse cortical neurons
- PMID: 34077150
- PMCID: PMC8327978
- DOI: 10.1503/jpn.200185
Chronic lithium treatment alters the excitatory/ inhibitory balance of synaptic networks and reduces mGluR5-PKC signalling in mouse cortical neurons
Abstract
Background: Bipolar disorder is characterized by cyclical alternation between mania and depression, often comorbid with psychosis and suicide. Compared with other medications, the mood stabilizer lithium is the most effective treatment for the prevention of manic and depressive episodes. However, the pathophysiology of bipolar disorder and lithium’s mode of action are yet to be fully understood. Evidence suggests a change in the balance of excitatory and inhibitory activity, favouring excitation in bipolar disorder. In the present study, we sought to establish a holistic understanding of the neuronal consequences of lithium exposure in mouse cortical neurons, and to identify underlying mechanisms of action.
Methods: We used a range of technical approaches to determine the effects of acute and chronic lithium treatment on mature mouse cortical neurons. We combined RNA screening and biochemical and electrophysiological approaches with confocal immunofluorescence and live-cell calcium imaging.
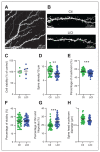
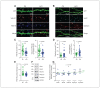
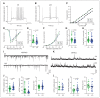
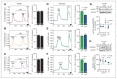
Results: We found that only chronic lithium treatment significantly reduced intracellular calcium flux, specifically by activating metabotropic glutamatergic receptor 5. This was associated with altered phosphorylation of protein kinase C and glycogen synthase kinase 3, reduced neuronal excitability and several alterations to synapse function. Consequently, lithium treatment shifts the excitatory–inhibitory balance toward inhibition.
Limitations: The mechanisms we identified should be validated in future by similar experiments in whole animals and human neurons.
Conclusion: Together, the results revealed how lithium dampens neuronal excitability and the activity of the glutamatergic network, both of which are predicted to be overactive in the manic phase of bipolar disorder. Our working model of lithium action enables the development of targeted strategies to restore the balance of overactive networks, mimicking the therapeutic benefits of lithium but with reduced toxicity.
© 2021 CMA Joule Inc. or its licensors
Conflict of interest statement
None declared.
Figures

Similar articles
-
Lithium, Stress, and Resilience in Bipolar Disorder: Deciphering this key homeostatic synaptic plasticity regulator.J Affect Disord. 2018 Jun;233:92-99. doi: 10.1016/j.jad.2017.12.026. Epub 2017 Dec 22. J Affect Disord. 2018. PMID: 29310970 Review.
-
Potential mechanisms of action of lithium in bipolar disorder. Current understanding.CNS Drugs. 2013 Feb;27(2):135-53. doi: 10.1007/s40263-013-0039-0. CNS Drugs. 2013. PMID: 23371914 Review.
-
Abnormalities in protein kinase C signaling and the pathophysiology of bipolar disorder.Bipolar Disord. 1999 Dec;1(2):81-6. doi: 10.1034/j.1399-5618.1999.010204.x. Bipolar Disord. 1999. PMID: 11252663 Review.
-
Lithium reduces the span of G protein-activated K+ (GIRK) channel inhibition in hippocampal neurons.Bipolar Disord. 2017 Nov;19(7):568-574. doi: 10.1111/bdi.12536. Epub 2017 Sep 12. Bipolar Disord. 2017. PMID: 28895268
-
Modulation of CNS signal transduction pathways and gene expression by mood-stabilizing agents: therapeutic implications.J Clin Psychiatry. 1999;60 Suppl 2:27-39; discussion 40-1, 113-6. J Clin Psychiatry. 1999. PMID: 10073385 Review.
Cited by
-
Monoallelic loss-of-function variants in GSK3B lead to autism and developmental delay.Mol Psychiatry. 2025 May;30(5):1952-1965. doi: 10.1038/s41380-024-02806-z. Epub 2024 Oct 29. Mol Psychiatry. 2025. PMID: 39472663
-
USP19 deubiquitinase inactivation regulates α-synuclein ubiquitination and inhibits accumulation of Lewy body-like aggregates in mice.NPJ Parkinsons Dis. 2023 Nov 28;9(1):157. doi: 10.1038/s41531-023-00601-1. NPJ Parkinsons Dis. 2023. PMID: 38017009 Free PMC article.
-
Lithium Biological Action Mechanisms after Ischemic Stroke.Life (Basel). 2022 Oct 22;12(11):1680. doi: 10.3390/life12111680. Life (Basel). 2022. PMID: 36362835 Free PMC article. Review.
-
New Advances in the Pharmacology and Toxicology of Lithium: A Neurobiologically Oriented Overview.Pharmacol Rev. 2024 May 2;76(3):323-357. doi: 10.1124/pharmrev.120.000007. Pharmacol Rev. 2024. PMID: 38697859 Free PMC article. Review.
-
The Mood Stabilizer Lithium Slows Down Synaptic Vesicle Cycling at Glutamatergic Synapses.Neuromolecular Med. 2023 Mar;25(1):125-135. doi: 10.1007/s12017-022-08729-8. Epub 2022 Nov 27. Neuromolecular Med. 2023. PMID: 36436129
References
-
- Angst J. The emerging epidemiology of hypomania and bipolar II disorder. J Affect Disord 1998;50:143–51. - PubMed
-
- Moreira ALR, Van Meter A, Genzlinger J, et al. . Review and meta-analysis of epidemiologic studies of adult bipolar disorder. J Clin Psychiatry 2017;78:e1259–69. - PubMed
-
- Vieta E, Berk M, Schulze TG, et al. . Bipolar disorders. Nat Rev Dis Primers 2018;4:18008. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
